Process for removing phosphorus from phosphorus water by using quantitative crystalline limestone and gypsum
A technology that is rich in phosphorus and water body, applied in the field of water pollution control, can solve the problems of high operating cost, high cost and harsh operating conditions, and achieve the effect of convenient use and water body optimization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
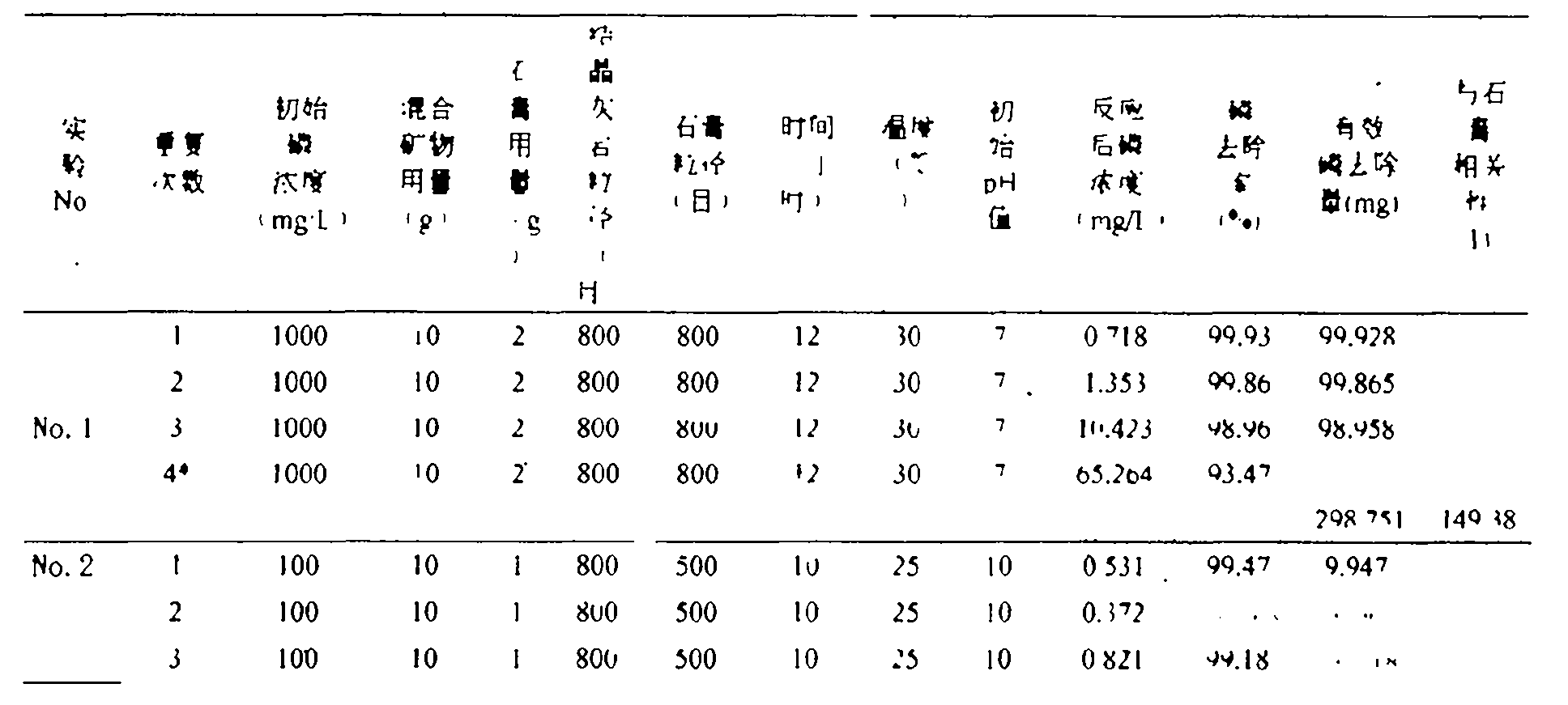
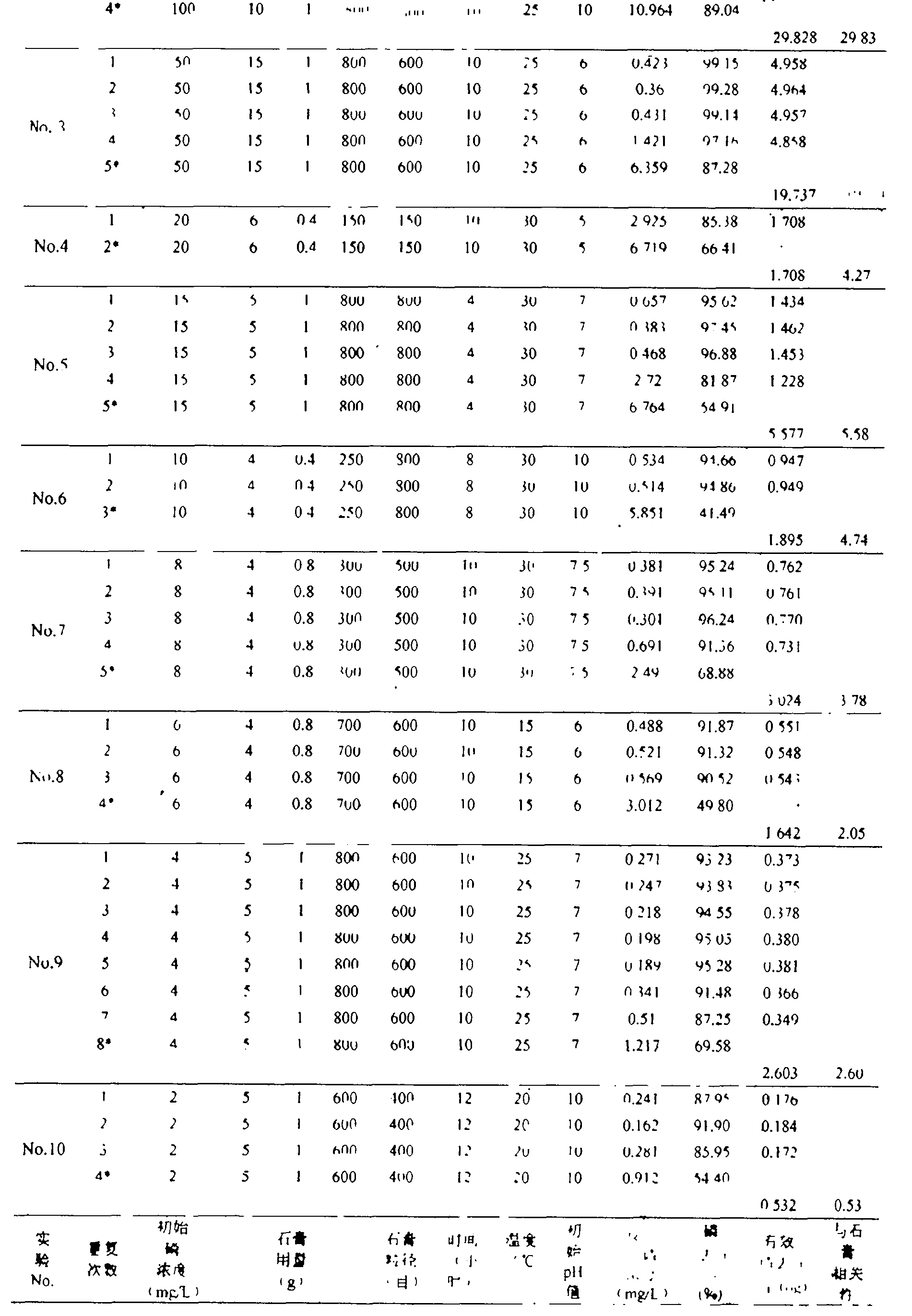
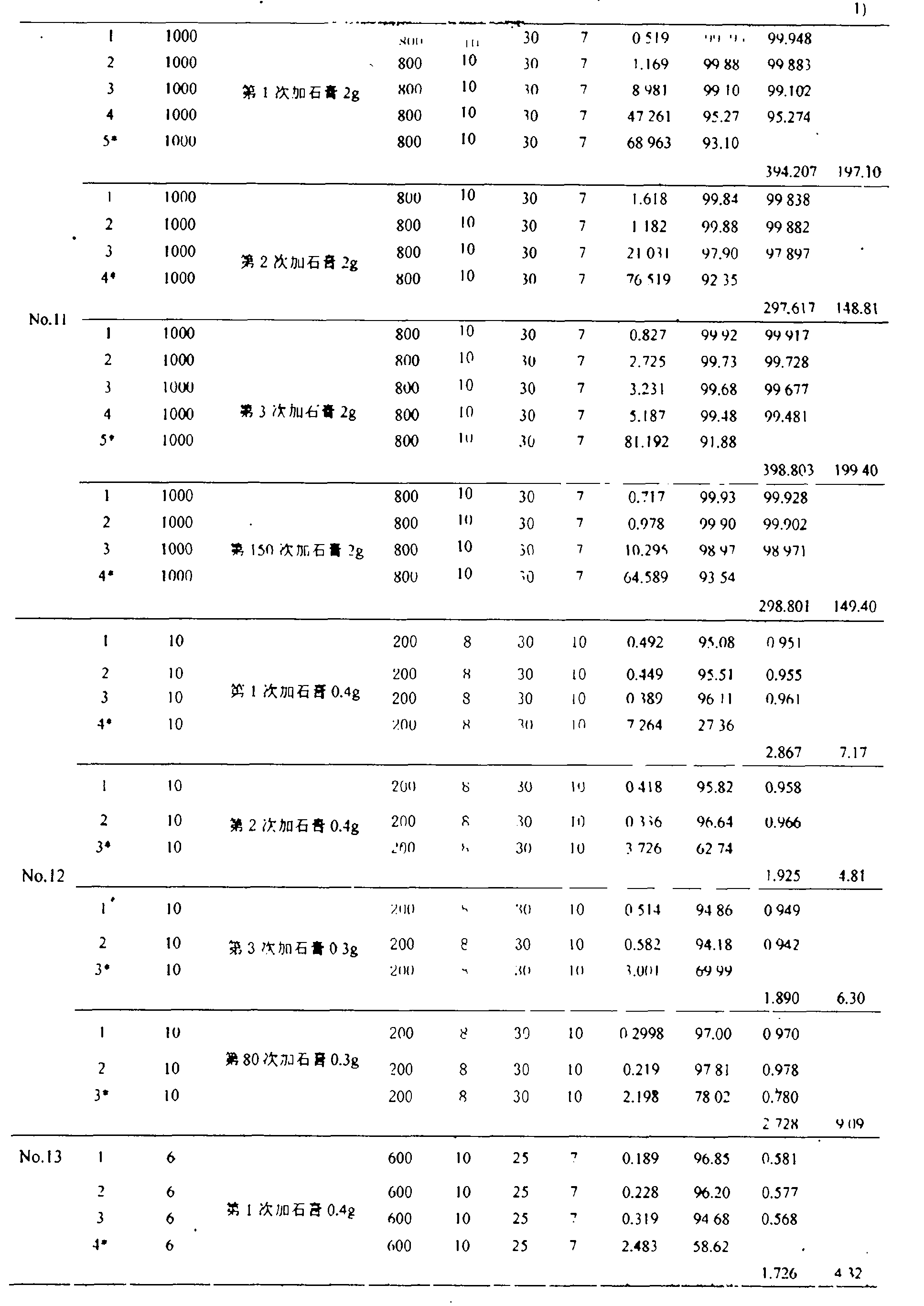
[0025] Take 10g of mixed mineral powder with a ratio of 4:1 (mass ratio of crystalline limestone / anhydrite) (the particle size of crystalline limestone is 800 mesh, and the particle size of gypsum is 800 mesh), and put it into a 200mL Erlenmeyer flask Add 100 mL of a solution with an initial phosphorus concentration of 1000 mg / L, adjust the pH to 7, put it into a constant temperature oscillator, set the rotation speed at 50 rpm, and the temperature at 30 ° C. After 12 hours of reaction, take its supernatant test. Then, remove the supernatant in the Erlenmeyer flask, dry the residual mixed mineral powder in the Erlenmeyer flask, then add 100 mL of a solution with an initial phosphorus concentration of 1000 mg / L, and repeat the experiment under the above conditions until it removes phosphorus (Recovery of phosphorus) until the effect is low. The final total amount of effective phosphorus removal is 298.75 mg, and the effective phosphorus removal amount corresponding to 1 g of g...
Embodiment 2
[0027] Take 10g of mixed mineral powder with a ratio of 9:1 (mass ratio of crystalline limestone / anhydrite) (the particle size of crystalline limestone is 800 mesh, and the particle size of gypsum is 500 mesh), and put it into a 200mL Erlenmeyer flask Add 100mL of a solution with an initial phosphorus concentration of 100g / L, adjust the pH to 6, put it into a constant temperature oscillator, set the rotation speed at 150 rpm, and the temperature at 25°C. After 10 hours of reaction, take its supernatant test. Then, remove the supernatant in the Erlenmeyer flask, dry the residual mixed mineral powder in the Erlenmeyer flask, then add 100 mL of a solution with an initial phosphorus concentration of 100 mg / L, and repeat the experiment under the above conditions until it removes phosphorus (Recovery of phosphorus) until the effect is low. The final total amount of effective phosphorus removal is 29.82 mg, and the effective phosphorus removal amount corresponding to 1 g of gypsum i...
Embodiment 3
[0029] Take 15g of mixed mineral powder with a ratio of 14:1 (mass ratio of crystalline limestone / anhydrite) (the particle size of crystalline limestone is g00 mesh, and the particle size of gypsum is 600 mesh), and put it into a 200mL conical flask Add 100mL of a solution with an initial phosphorus concentration of 50mg / L, adjust the pH to 6, put it into a constant temperature oscillator, set the speed at 150 rpm, and the temperature at 25°C. After 10 hours of reaction, take the supernatant test. Then, remove the supernatant in the Erlenmeyer flask, dry the residual mixed mineral powder in the Erlenmeyer flask, then add 100 mL of a solution with an initial phosphorus concentration of 50 mg / L, and repeat the experiment under the above conditions until it removes phosphorus (Recovery of phosphorus) until the effect is low. The final total amount of effective phosphorus removal is 19.76 mg, and the effective phosphorus removal amount corresponding to 1 g of gypsum is 19.76 mg (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com