Qualitative and quantitative detection dual-purpose HCG test paper for colloidal gold immunochromatography assay
A technology for detecting test strips and immunochromatography, which is applied in the field of in vitro immunodiagnostic reagents to achieve the effects of small coefficient of variation, high detection accuracy, and avoiding inconvenience and errors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 human chorionic gonadotropin (HCG) immunochromatography test paper
[0049] 1. Preparation of colloidal gold-labeled HCG monoclonal antibody complex
[0050] Label the HCG monoclonal antibody on the surface of the prepared colloidal gold particles with an average particle size of 3nm-80nm, wash with 0.002M boric acid buffer (containing 1.0% sucrose, 1% BSA) and concentrate to 1 / 2 of the original volume -1 / 50, generally 1 / 10, stored at 4°C.
[0051] 2. Preparation of immunochromatographic test paper
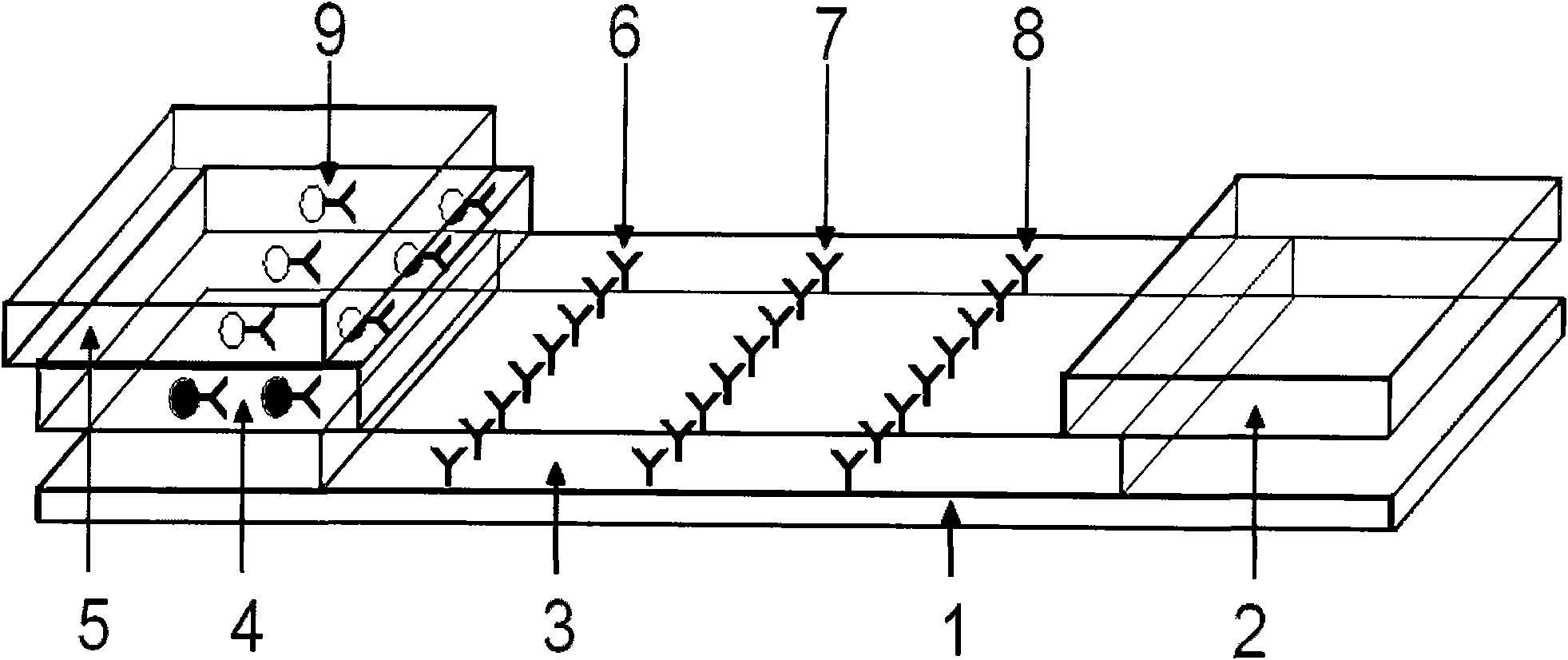
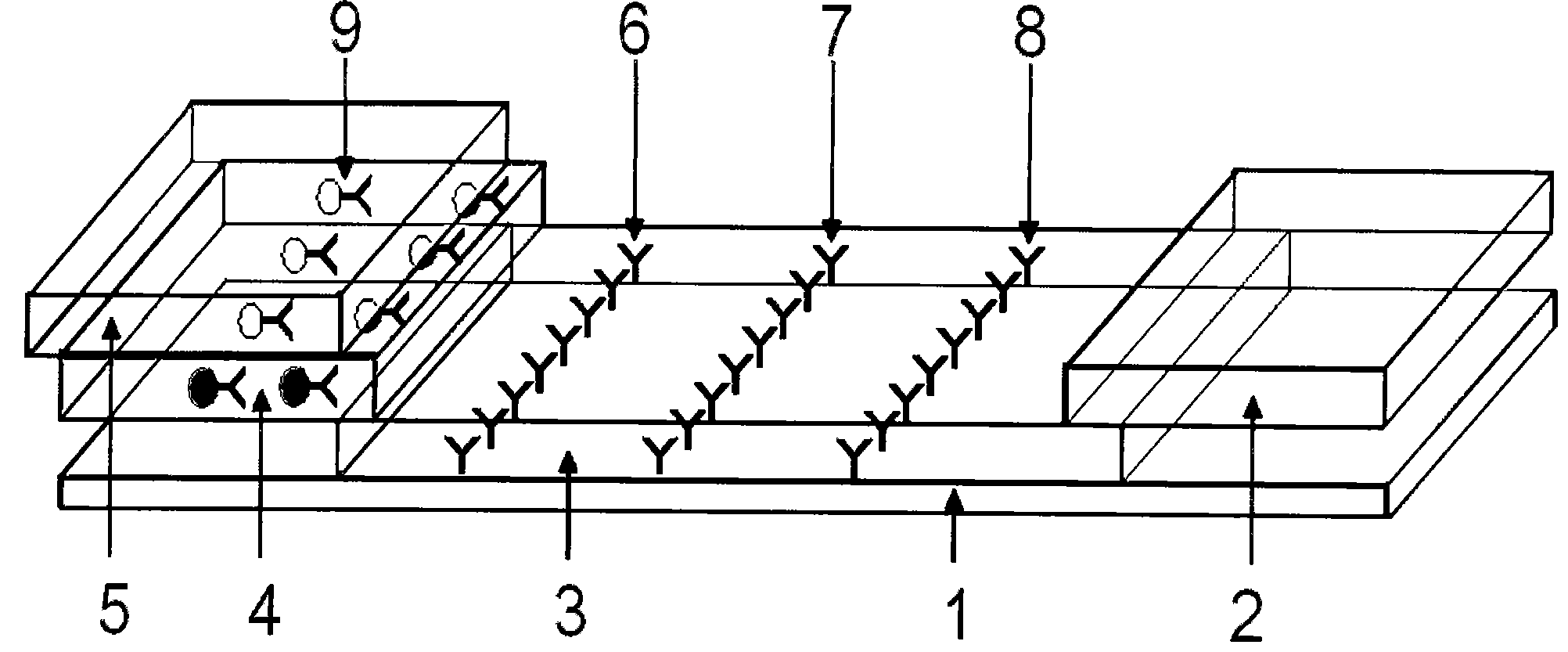
[0052] 2.1 Preparation of sample pad: select glass cellulose membrane as the sample pad material, soak it in the sample pad treatment solution (0.01mol / LPBS (pH=7.4), containing 1% BSA, 0.1% Triton X-100), Dry at 30-42°C, generally at 37°C for later use.
[0053] 2.2 Preparation of conjugation pad: glass cellulose membrane was selected as the conjugation pad material, and the colloidal gold-HCG antibody complex prepared above was redispers...
Embodiment 2
[0056] Example 2 Qualitative detection of 60 clinical patient urine samples and result judgment
[0057] Take 50 positive urine samples and 10 negative urine samples as the samples to be tested, drop them into the sample holes of the test card respectively, and let it stand for 10 minutes. As a result, 23 samples appear red at the qualitative line and the quality control line. It is a positive sample; 27 samples all appear red in the three lines of qualitative line, quality control line and quantitative line, which are strong positive samples; 10 samples only appear red in the quality control band, which is a negative sample (HCG concentration is less than 25IU / L). In the test, if the quality control strip does not develop color, it is invalid.
Embodiment 3
[0058] Example 3 Quantitative detection of 25 clinical patient samples
[0059] 1. Use normal human urine as the diluent for the standard solution of HCG antigen, and configure a series of concentration standard products as follows: 0IU / L, 25IU / L, 100IU / L, 300IU / L, 500IU / L, 800IU / L, 1000IU / L, 3000IU / L, 5000IU / L, 8000IU / L, 10000IU / L, and 15000IU / L.
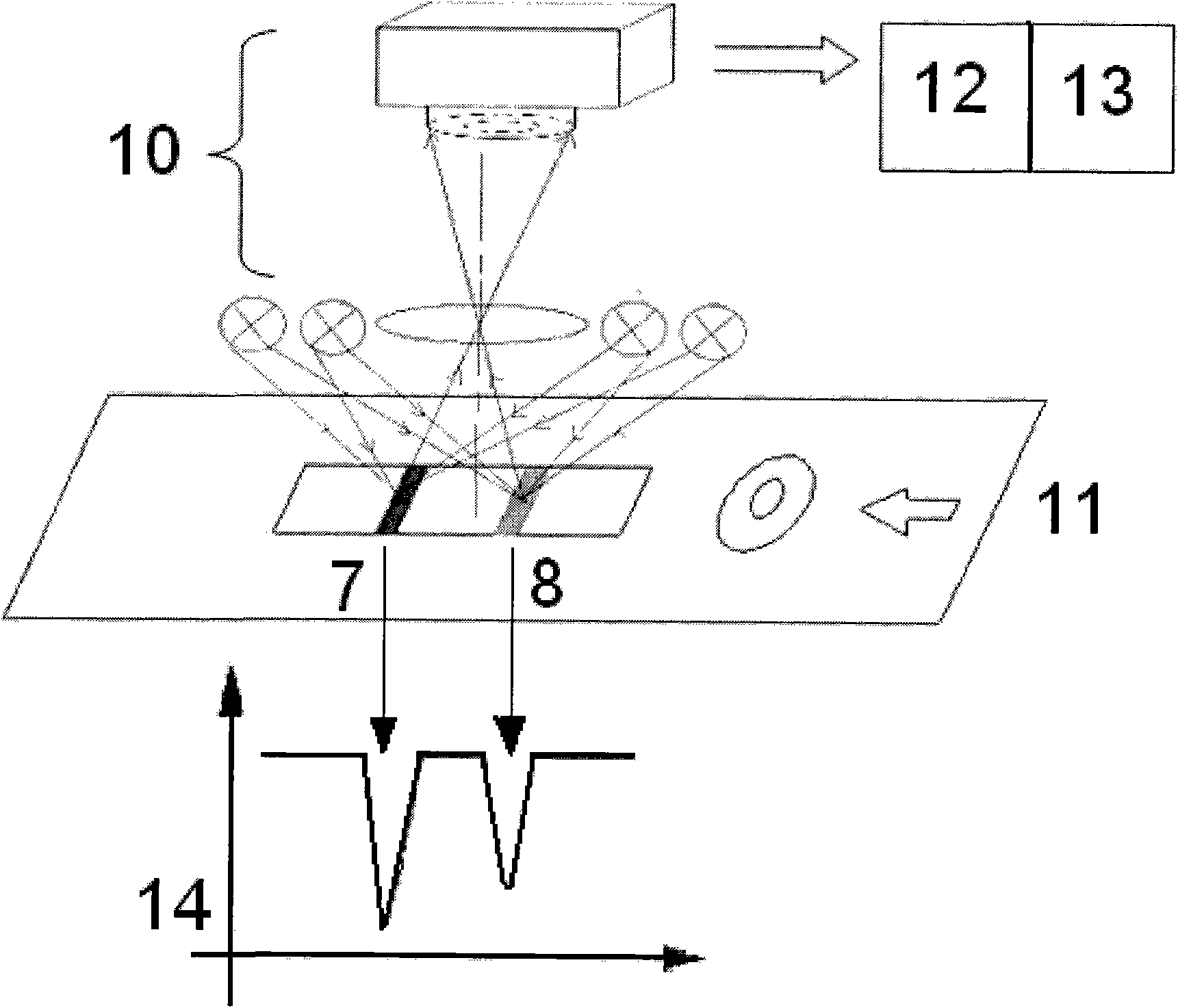
[0060] 2. Add the standard substance to be tested dropwise into the sampling hole of the detection card, and read the ratio of the signal intensity of the quantitative line to the quality control line through a quantitative detector after 10 minutes (each sample is measured 3 times with 3 detection cards, Take the average value) and draw the corresponding standard curve.
[0061] 3. Use the above standard curve to set the built-in standard curve of the immunochromatography quantitative detector.
[0062] 4. Detect 25 clinical patient samples according to the method in the above step 2, and obtain the content of HCG in the clinic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com