Method for preparing 1-benzyl-4- benzylthiomethyl-pyridinium iodide
A technology of benzylthio and pyridinium salts, which is applied in the field of preparation of 1-benzyl-4-thiobenzyl-pyridinium salt iodide, which can solve the strong toxicity of benzyl halides, the difficulty of obtaining synthetic raw materials, and the strong corrosion of benzylamine To achieve the effect of high product purity, low cost and reduced toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
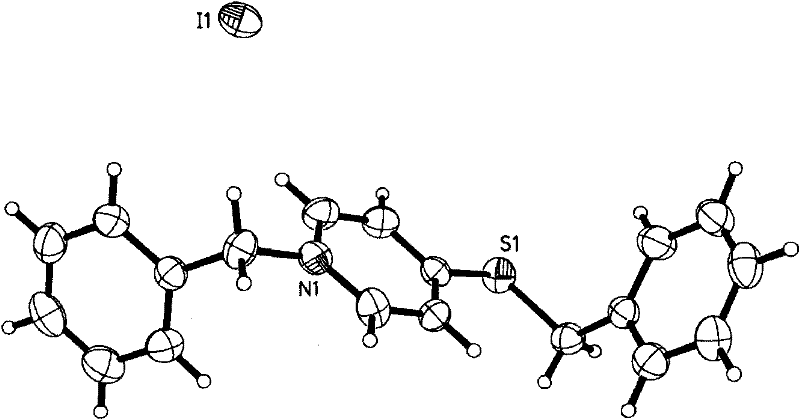
[0039] Embodiment one, see appendix figure 1 , the preparation method of 1-benzyl-4-thiobenzyl-pyridinium iodide, comprises the following steps:
[0040] (1) 0.10 mmol iodine (I 2 ), 0.10 mmol of 4-mercaptopyridine, 1.0 mmol of benzyl alcohol and 3 microliters of water were mixed in 2 milliliters of acetonitrile, and reacted for 1000 to 7000 minutes at 100° C. to 150° C. under solvothermal reaction conditions. The preferred condition is to react for 2000 minutes under solvothermal reaction conditions of 150°C.
[0041] (2) Then slowly lower the temperature to obtain an orange-red mother liquor containing 1-benzyl-4-thiobenzyl-pyridinium iodide. The mother liquor was mixed with 10 ml of diethyl ether to obtain a light pink crystalline product of 1-benzyl-4-thiobenzyl-pyridinium iodide. If 10 milliliters of ether is carefully covered on the mother liquor, and the two liquid phases are slowly diffused with each other, a purple-red long flaky single crystal of 1-benzyl-4-thiobe...
Embodiment 2
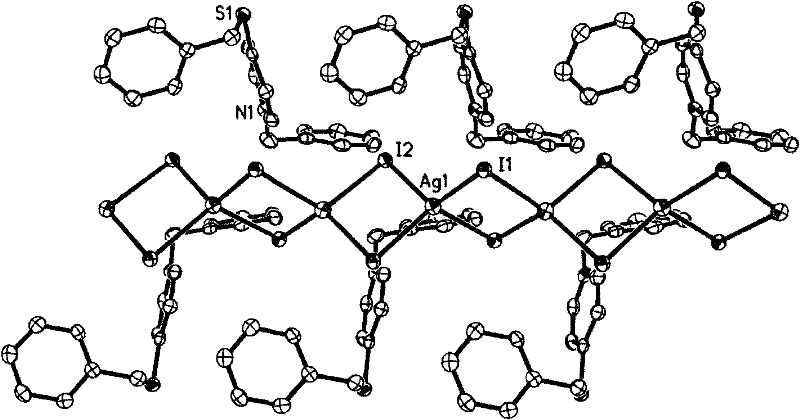
[0045] Embodiment two: see attached figure 2 , using 1-benzyl-4-thiobenzyl-pyridinium iodide for the synthesis of functional metal complexes, comprising the following steps:
[0046] (1) Mix 0.10 mmol of silver iodide, 0.10 mmol of potassium iodide and 0.10 mmol of 1-benzyl-4-thiobenzyl-pyridinium iodide in 2 ml of acetonitrile, and react for 2000 minutes at 150°C under solvothermal reaction conditions ;
[0047] (2) then slowly cool down to obtain yellow needle-like crystals {[(C19 h 18 NS)(AgI 2 )] n}. Its single crystal structure is figure 2 As shown, the anion part is one-dimensional (AgI 2 ) n chain.
[0048] (3) the crystalline product is enriched by filtration, washed three times with two milliliters of ethyl acetate respectively, and dried at normal temperature to obtain high-purity crystalline {[(C 19 h 18 NS)(AgI 2 )] n}product. Yield: 66%.
[0049] (4) Elemental analysis: theoretical value (C 19 h 18 NSAgI 2 ): C, 34.89; H, 2.77; N, 2.14. Found: ...
Embodiment 3
[0051] Embodiment three, see appendix figure 1 , the preparation method of 1-benzyl-4-thiobenzyl-pyridinium iodide, comprises the following steps:
[0052] (1) 0.10 mmol iodine (I 2 ), 0.10 mmol of 4-mercaptopyridine, 1.0 mmol of benzyl alcohol and 3 microliters of water were mixed in 2 milliliters of acetonitrile, and reacted for 3000 minutes at 140 ° C under solvothermal reaction conditions.
[0053] (2) Then slowly lower the temperature to obtain an orange-red mother liquor containing 1-benzyl-4-thiobenzyl-pyridinium iodide. The mother liquor was mixed with 10 ml of diethyl ether to obtain a light pink crystalline product of 1-benzyl-4-thiobenzyl-pyridinium iodide. If 10 milliliters of ether is carefully covered on the mother liquor, and the two liquid phases are slowly diffused with each other, a purple-red long flaky single crystal of 1-benzyl-4-thiobenzyl-pyridinium salt iodide can be obtained after one week. Its single crystal structure (C 19 h 18 NS) (I) such as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com