Preparation method for novel fusion protein reversing renal fibrosis and activity detection
A fusion protein and activity detection technology, applied in the field of medicine and biology, can solve the problems of high dosage of BMP7, safety problems, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
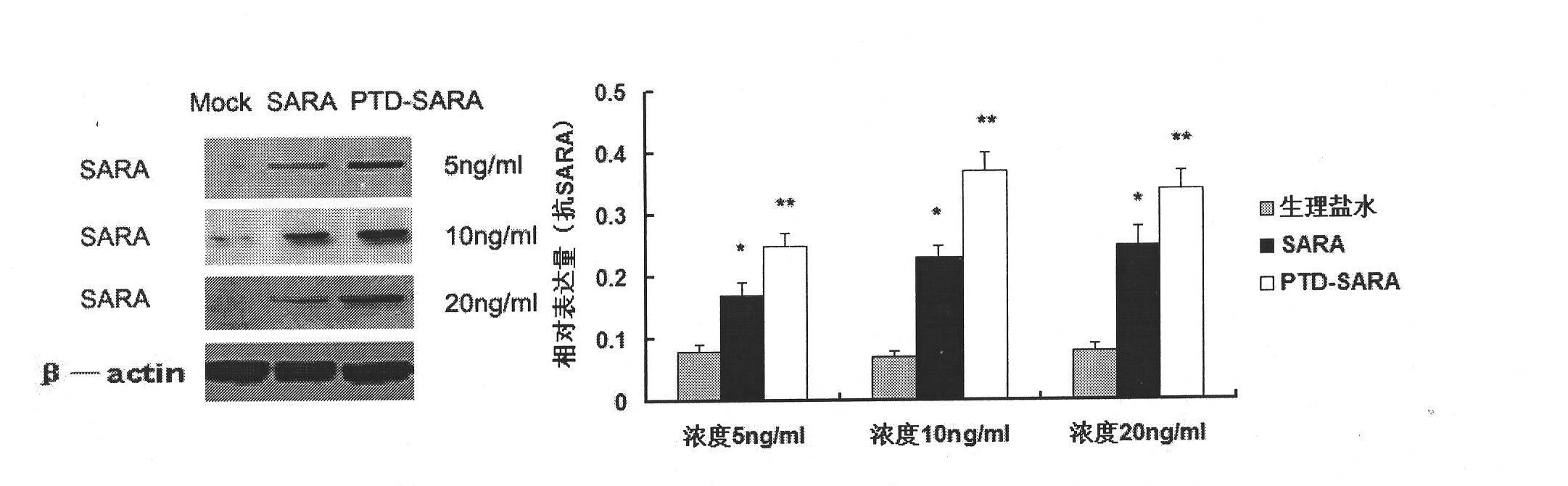
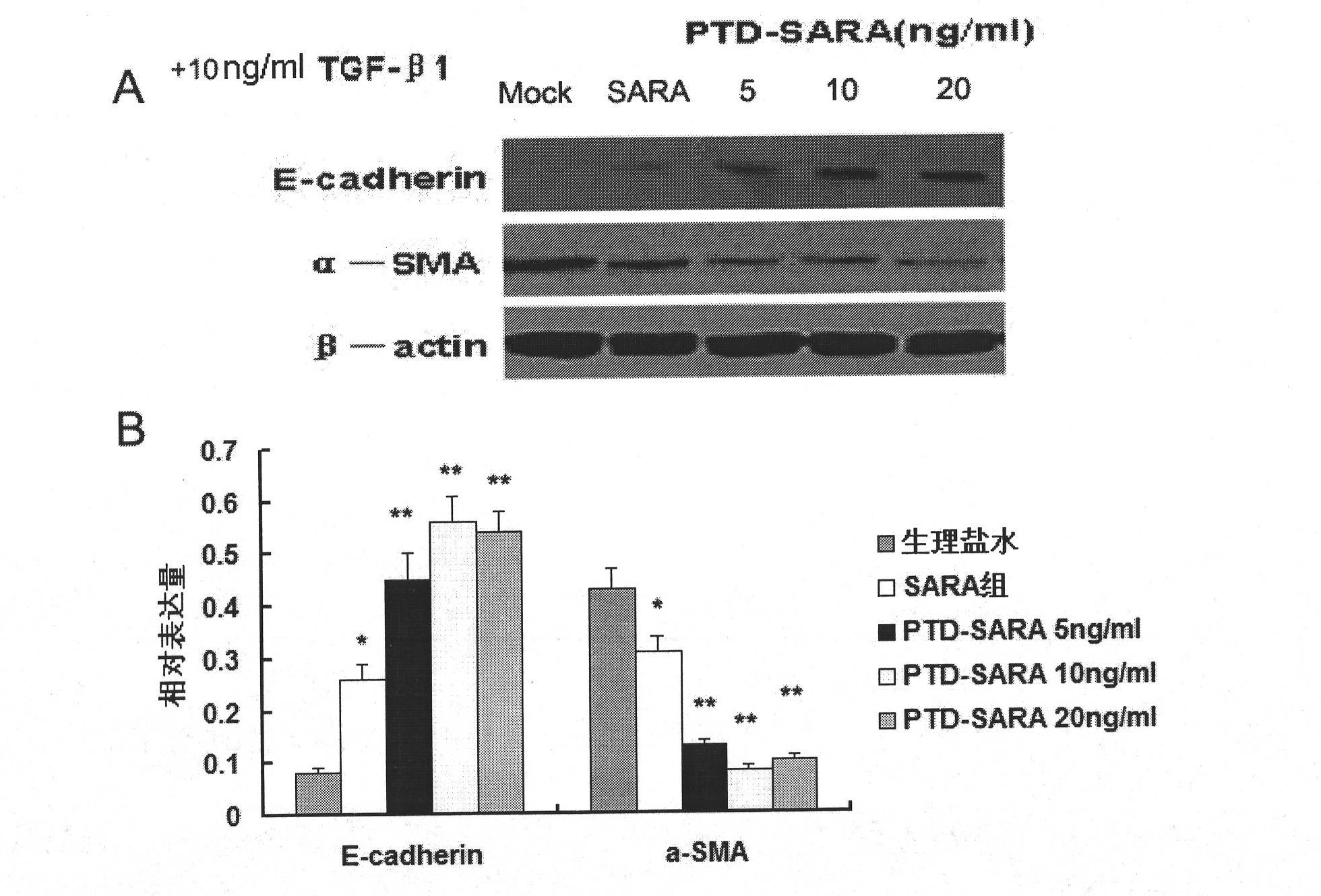
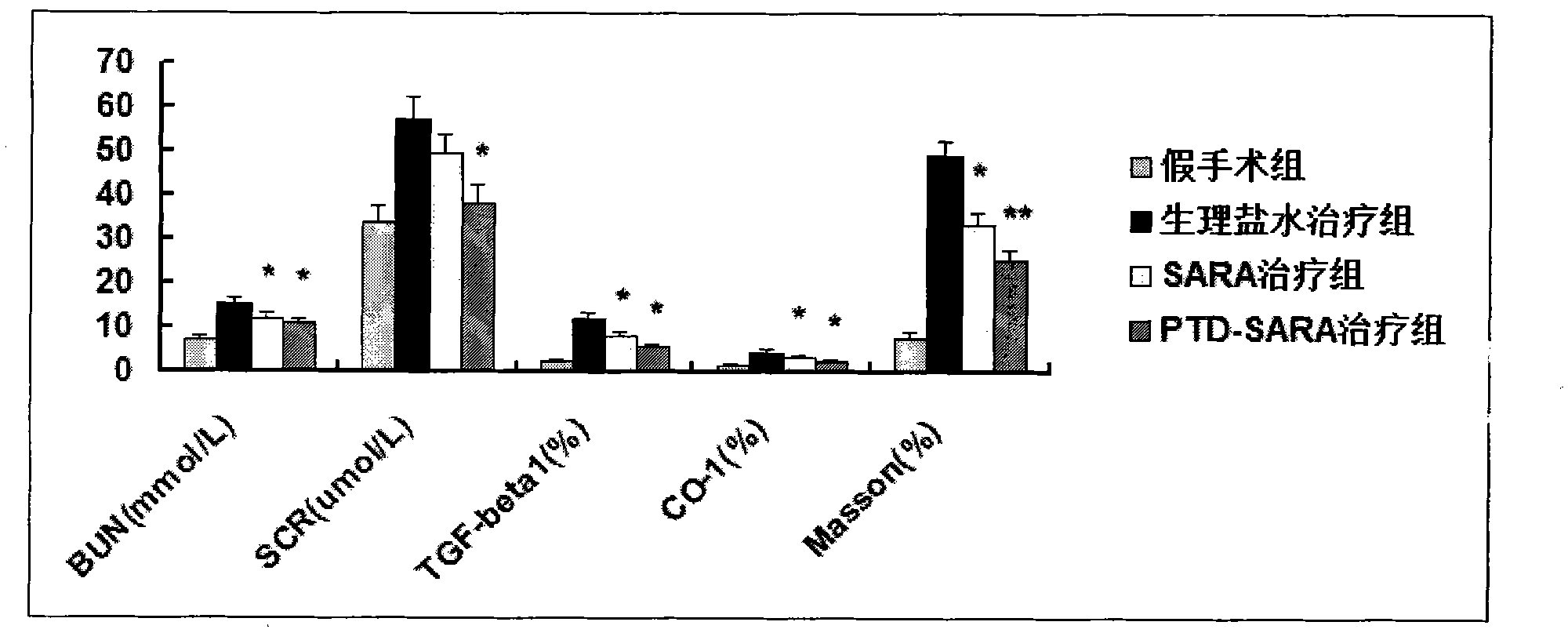
[0042] The present invention will be described in further detail below in conjunction with the accompanying drawings of the embodiments. It should be noted that the following examples are only used to illustrate the present invention and are not intended to limit the scope of the present invention.
[0043] Implemented the full sequence determination of a new type of transmembrane fusion protein that inhibits the TGF-β1 pathway and reverses renal fibrosis. The results of PGE-PTD-SARA sequencing are:
[0044] GGATCC TAT GCT CGT GCC GCA GCG CGG CAA GCT CGT GCT GGT CGG AAGTGG TGG CGG AAG TGG TGG CGG AAG TAT GAG CGC GGC CAG AGC CCG AACCCG AAC AAC CCG GCG GAA TAT TGC AGC ACC ATT CCG CCG CTG CAG CAGGCG CAG GCG AGC GGC GCG CTG AGC AGC CCG CCG CCG ACC GTG ATG GTGCCG GTG GGC GTG CTG AAA CAT CCG GGC GCG GAA GTG GCG CAG CCG CGTTAG GTCGAC
[0045] The method for preparing the above-mentioned novel penetrating fusion protein that inhibits TGF-β1 pathway and reverses renal fibrosis is as f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com