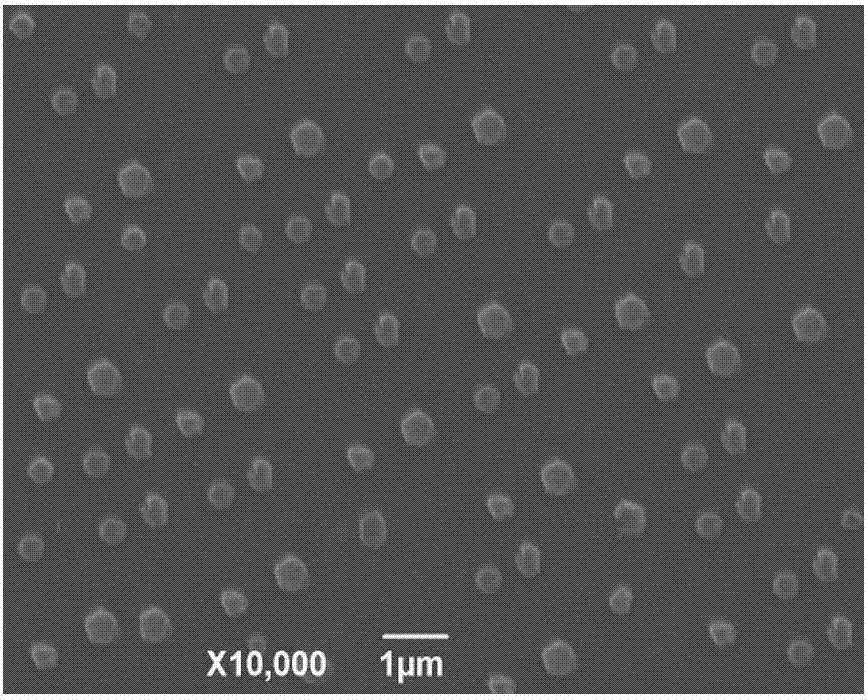
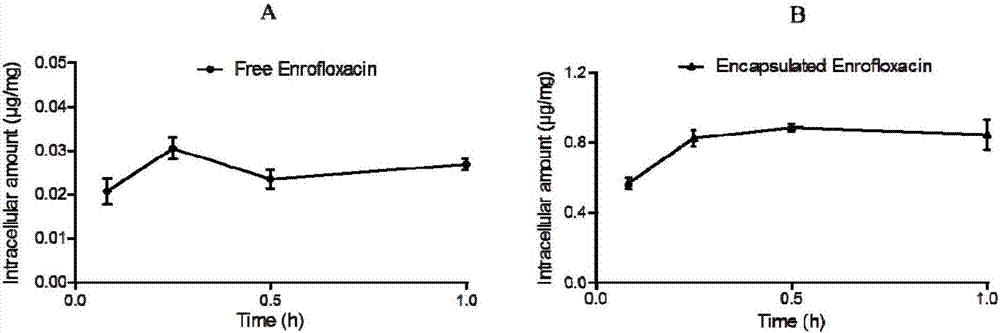
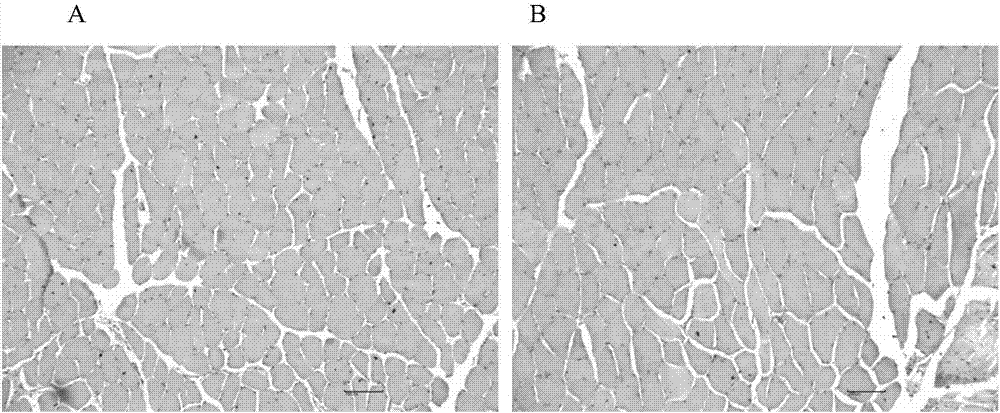
Veterinary enrofloxacin solid lipid nanosuspension and preparation method thereof
A technology of solid lipid nanometer and enrofloxacin, which is applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, and liquid delivery, etc. Low drug dosage and other problems, to achieve the effect of reducing clinical drug dosage, improving absorption, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The formula of enrofloxacin solid lipid nanosuspension for veterinary use is shown in Table 1, and the preparation method is as follows:
[0060] (1) Weigh 12g of behenic acid in a test tube heated in an oil bath at 120°C, add 2g of enrofloxacin raw material to it after heating to melt, and form a transparent enrofloxacin solution under constant stirring;
[0061] (2) Weigh 0.2g of sodium lauryl sulfate and sodium bisulfite and add them to 30mL of sterilized water to make a solution;
[0062] (3) The solution prepared in step (2) is heated in an oil bath at 120°C and added to the enrofloxacin solution prepared in step (1) at a stirring speed of 3000-5000 r / min to form an oil-in-water colostrum;
[0063] (4) Disperse the colostrum prepared in step (3) for 2 to 5 minutes to form an oil-in-water emulsion;
[0064] (5) Quickly pour the emulsion prepared in step (4) into 30 mL of sterilized water at 4° C. to obtain a suspension of enrofloxacin particles;
[0065] (6) Use a high-pressu...
Embodiment 2
[0070] The formula of enrofloxacin solid lipid nanosuspension for veterinary use is shown in Table 2. The preparation method is as follows:
[0071] (1) Weigh 12g of behenyl alcohol into a test tube heated in an oil bath at 120°C, add 2g of enrofloxacin raw material to it after heating to melt, and form a transparent enrofloxacin solution under constant stirring;
[0072] (2) Weigh 0.5g poloxamer 188, 0.2g sodium sulfoxylate formaldehyde and 3.5g dimethyl dioctadecyl ammonium chloride and add them to 30mL sterile water to make a solution;
[0073] (3) The solution prepared in step (2) is heated in an oil bath at 120°C and added to the enrofloxacin solution prepared in step (1) at a stirring speed of 3000-5000 r / min to form an oil-in-water colostrum;
[0074] (4) Disperse the colostrum prepared in step (3) for 2 to 5 minutes to form an oil-in-water emulsion;
[0075] (5) Quickly pour the emulsion prepared in step (4) into 20 mL of sterilized water at 4° C. to obtain a suspension of enro...
Embodiment 3
[0081] The formula of enrofloxacin solid lipid nanosuspension for veterinary use is shown in Table 3. The preparation method is as follows:
[0082] (1) Weigh 20g of behenyl alcohol and 15g of behenic acid into a test tube heated in an oil bath at 120°C, add 6g of enrofloxacin raw material to it after heating to melt, and form transparent enrosha under constant stirring. Star solution
[0083] (2) Weigh 2g polyvinylpyrrolidone K separately 15 Add 1g formaldehyde and sodium hyposulfite to 40mL sterile water to make a solution;
[0084] (3) The solution prepared in step (2) is heated in an oil bath at 120°C and added to the enrofloxacin solution prepared in step (1) at a stirring speed of 3000-5000 r / min to form an oil-in-water colostrum;
[0085] (4) Disperse the colostrum prepared in step (3) for 2 to 5 minutes to form an oil-in-water emulsion;
[0086] (5) Quickly pour the emulsion prepared in step (4) into 15 mL of sterilized water at 4° C. to obtain a suspension of enrofloxacin par...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com