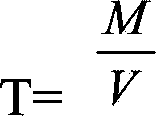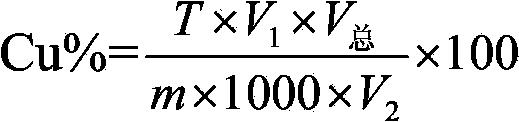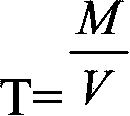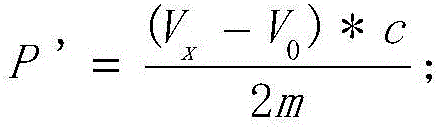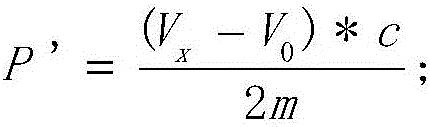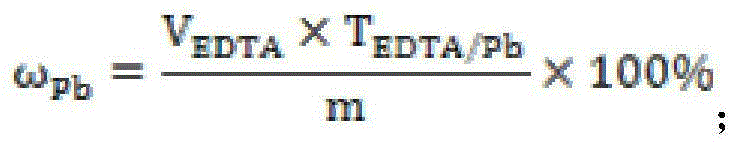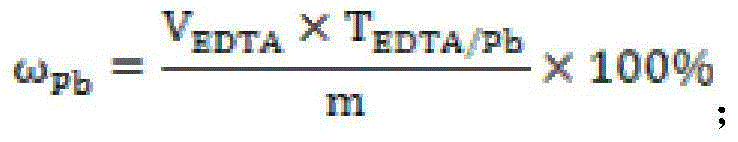Patents
Literature
180 results about "Sodium hyposulfite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sodium thiosulfate | sodium thiosulphate | Sodium hyposulfite [synonym] | Hyposulphite of soda [synonym] | Na2S2O3 [chemical formula] a colorless crystalline compound that is more familiar as the pentahydrate, Na2S2O3·5H2O, an efflorescent, monoclinic crystalline substance also called sodium hyposulfite or "hypo.".
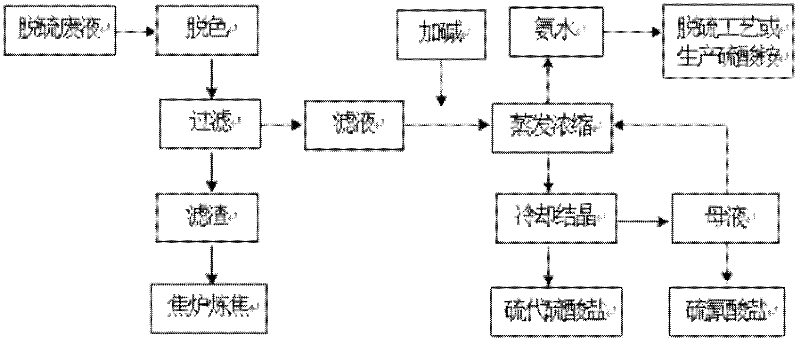
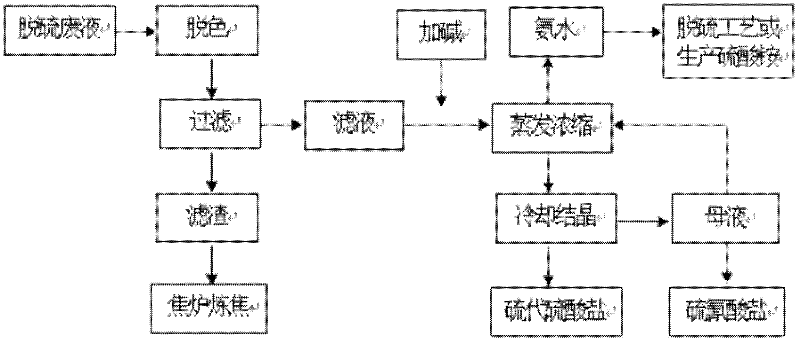
Method for treating desulfurization waste liquid of wet oxidation method
ActiveCN102295379AReduce manufacturing costNot easily decomposed by heatThiosulfates/dithionites/polythionitesThiocyanic acidSodium thiocyanateEvaporation
A method for treating desulfurization waste liquid of a wet oxidation method. The method comprises the following steps: firstly adding a decolorant into desulfurization waste liquid for decolorizing treatment, filtering after decolorization, adding alkali into the filtrate, controlling the PH to be 8-9, performing evaporation concentration under a condition with a negative pressure, condensing the evaporated ammonia gas and water vapor to obtain concentrated ammonia liquor which can be returned to the system for recycle or be used to produce ammonium sulfate, performing fractional crystallization of the concentrated desulfurization liquid to respectively obtain high-purity sodium thiocyanate and sodium hyposulfite. The invention has a simple process, and stable operations, and effectively solves the problem of difficult HPF desulfurization waste liquid treatment in coking enterprises; the method not only reduces the discharge of the waste liquid, but also recovers a lot of chemical products with economic value, and the method has quite significant economic benefits and environmental benefits.
Owner:SHOUGANG CORPORATION
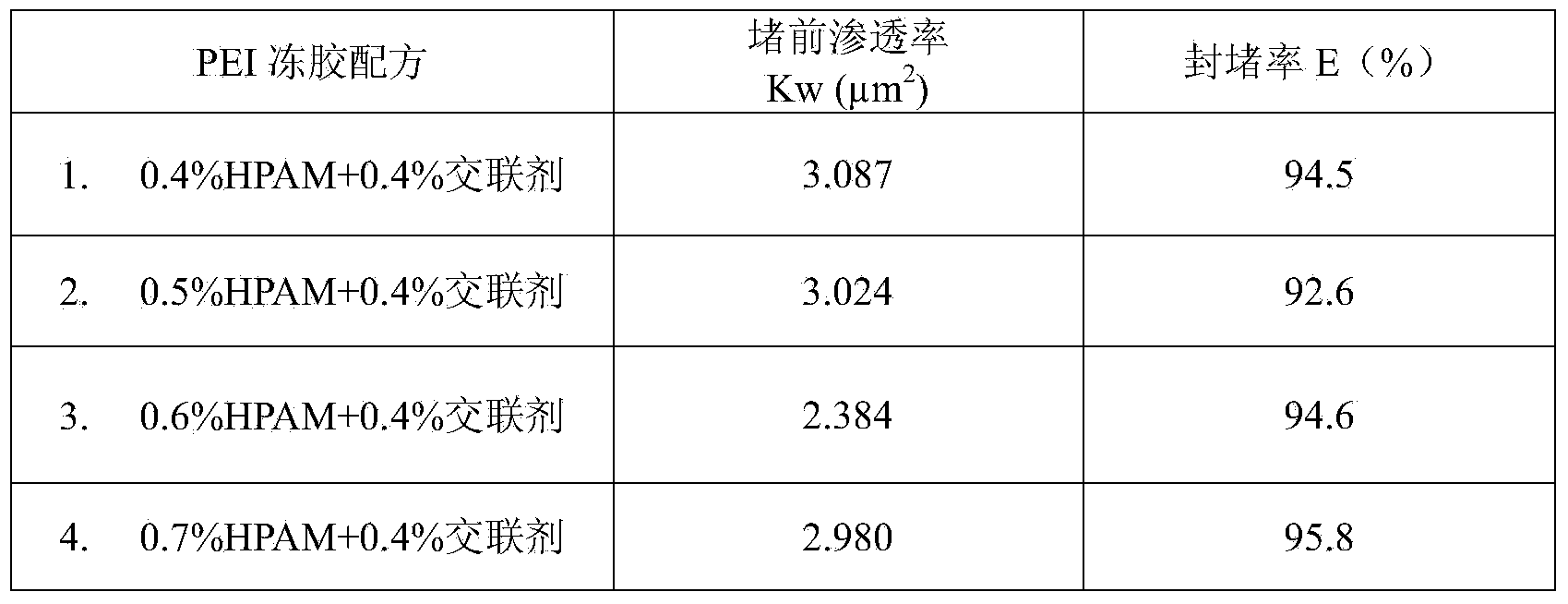
Polyethyleneimine jelly profile-control water-blocking agent
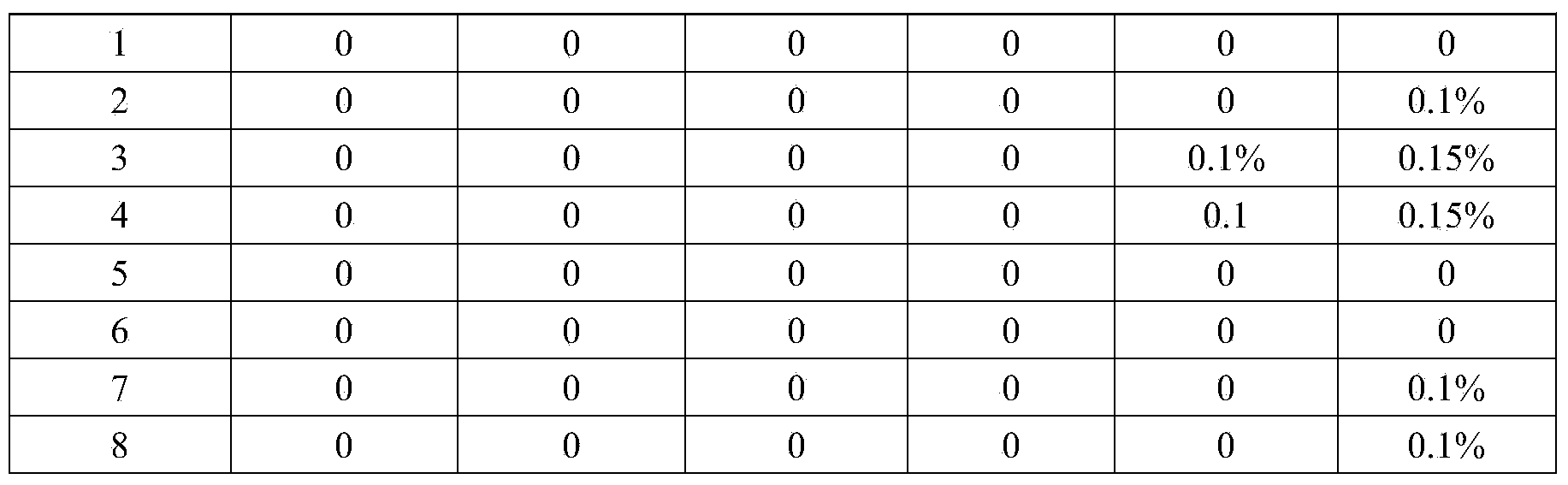
The invention provides a polyethyleneimine jelly profile-control water-blocking agent. The profile-control water-blocking agent is prepared by reacting partially-hydrolyzed polyacrylamide and a cross-linking agent polyethyleneimine. The profile-control water-blocking agent comprises following components by weight: 0.3-0.8% of the partially-hydrolyzed polyacrylamide, 0.2-0.5% of the cross-linking agent polyethyleneimine and 0.3-0.8% of an additive, with the balance being water, wherein the partially-hydrolyzed polyacrylamide adopts anionic polyacrylamide, and the additive is one compound selected from a group of sodium sulfite, sodium bisulfate, and sodium hyposulfite or thiourea, or is a mixture of several compounds selected from the group. The jelly forming time of the profile-control water-blocking agent is adjustable and the jelly has high strength. The profile-control water-blocking agent can block underground water having a temperature lower than 110 DEG C and a NaCl mineralization degree of 50000 mg / L. The profile-control water-blocking agent has good temperature toleration and cannot be dehydrated for 120 days.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA) +1
Electrolyte corrosion inhibitor for aluminum-air cell, electrolyte and preparation method
InactiveCN103633396AReduce hydrogen evolution self-corrosion rateProne to anodic polarizationFuel and primary cellsAluminum anodePhysical chemistry
The invention discloses an electrolyte corrosion inhibitor for an aluminum-air cell, an electrolyte and a preparation method, belonging to the technical field of chemical batteries. The electrolyte corrosion inhibitor mainly comprises sodium hyposulfite which has a concentration of 0.005 to 0.2 mol / L in the electrolyte and may further comprise the auxiliary additive sodium stannate which has a concentration of 0.01 to 0.03 mol / L in the electrolyte. The aluminum-air cell provided by the invention has the advantages of simple composition, low cost, safety and accordance with environmental protection requirements, can substantially reduce the hydrogen evolution self-corrosion rate of an aluminum anode, enables the open circuit potential of the aluminum anode and working potential under the condition of impressed current to undergo substantial negative transfer, allows an aluminum anode alloy to have good corrosion resistance and high electrochemical activity and meets the requirement of an alkaline aluminum-air cell for large current density discharging.
Owner:HENAN UNIV OF SCI & TECH
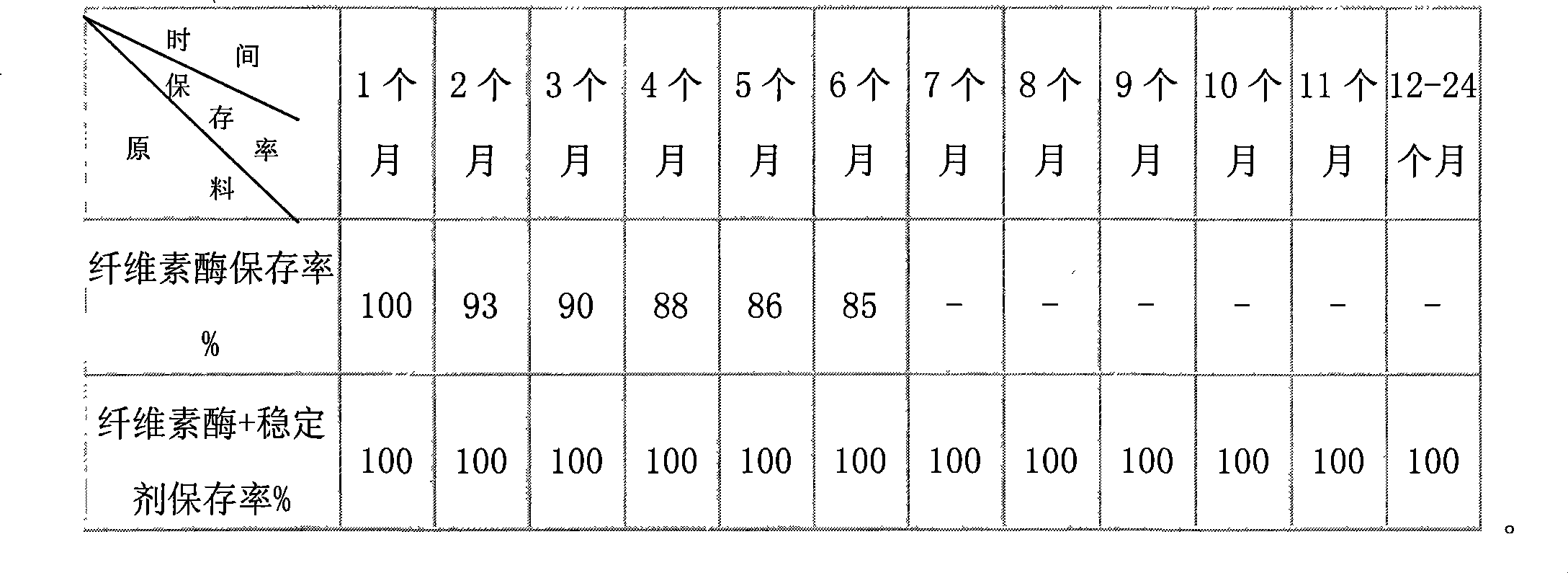
Composite stabilizer for preservation of liquid cellulase
The invention discloses a composite stabilizing agent for preserving a liquid cellulase, which can preserve the vitality of the cellulase for a long time under the liquid condition. The composite stabilizing agent is prepared from the raw materials including sucrose, sodium chloride, potassium sorbate and sodium hyposulfite by blending and stirring. The weight percentages of the raw materials in the liquid cellulase are as follows: 30 to 50 percent of the sucrose, 5 to15 percent of the sodium chloride, 0.01 to 0.03 percent of the potassium sorbate and 0.01 to 0.02 percent of the sodium hyposulfite. The composite stabilizing agent uses the sucrose and the sodium chloride so that cellulase protein molecules keep the stable structures easily in water molecules and are not easy to denaturalize and lose the activity, uses the potassium sorbate to control the colony count so as to meet the requirements on food conditions conveniently, and uses the sodium hyposulfite for preventing enzyme protein from being oxidized to lose the activity. The four raw materials of the composite stabilizing agent is easily available, the food grade is relatively high so that the cellulase activity cannot be reduced within two years at room temperature, the cost for building a refrigerated storage for keeping the cellulase activity is saved, and the preservation and transportation of the liquid cellulase are convenient.
Owner:ZHAODONG SUN SHINE ENZYME
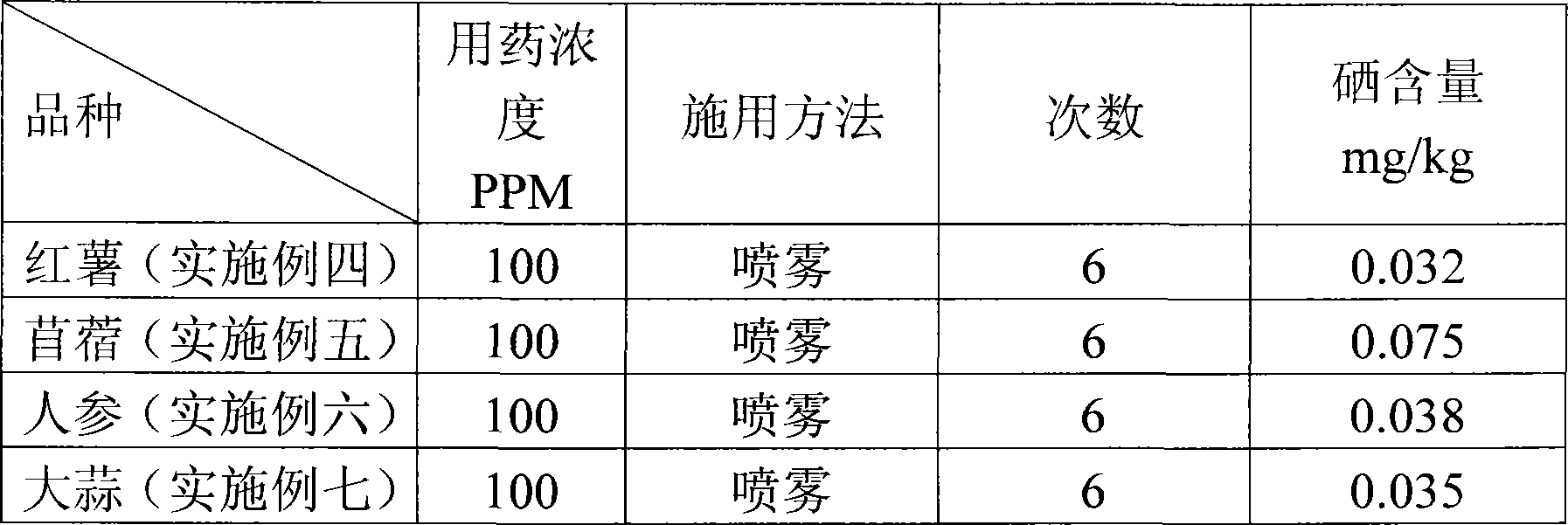
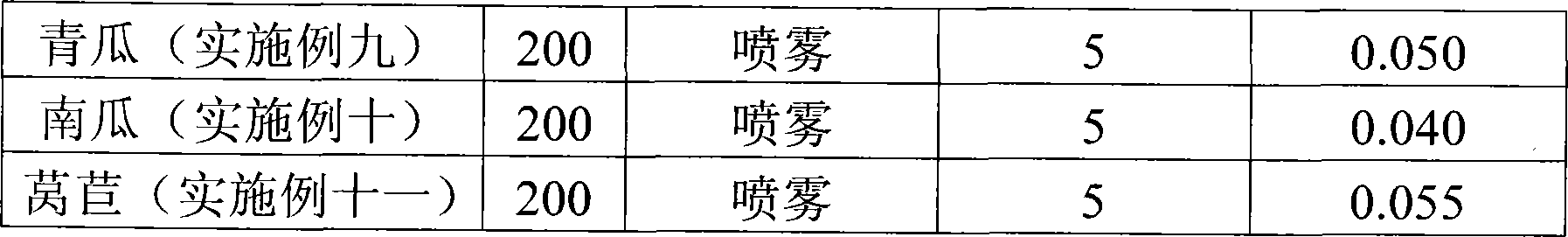
Selenium-enriched fertile solution and application method for selenium-enriched agricultural product production
InactiveCN101182246AIncrease selenium contentThe preparation method is simple and easySodium dithioniteInorganic selenium
A selenium-enriched fertilizer liquid is produced according to the steps as follows: coal ash granules are washed down from a coal flue by water; the obtained coal ash granules are aired, crushed and sieved through an eighty-mesh sieve; the coal ash after sieving is added into extract liquid which contains sodium hydroxide and sodium dithionite; sodium hyposulfite is added into the solution; the solution after being disposed by the sodium hyposulfite is put into a sedimentation barrel to be placed statically for 24 hours, and clear liquid at the upper layer is taken; the clear liquid at the upper layer which is obtained from last step is neutralized by vitriol, the pH value is adjusted to be 8 to 8.5, and the selenium-enriched fertilizer liquid is obtained. The method of applying the selenium-enriched fertilizer liquid to the production of the selenium-enriched agricultural products is that before the agricultural products are harvested, the selenium-enriched fertilizer liquid is diluted till 100ppm to 200ppm and sprayed at the agricultural products. The invention adopts the coal ash which is washed down from the coal flue by water to extract inorganic selenium, the manufacturing method of which is simple with very low cost, and the selenium content of the agricultural products can be improved effectively by spraying the manufactured selenium-enriched fertilizer liquid at the crop.
Owner:慈溪市蔬菜开发有限公司
Color development liquid for peroxidase mensuration and preparation method thereof
InactiveCN101354354AEliminate catalytic decompositionEasy to prepareMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementPeroxidaseTert butyl
The invention provides a visualization reagent architecture that is used for peroxydase measurement and a preparation method thereof. The visualization reagent architecture comprises urea peroxide, a heavy metal ion complexing agent and a compound of 4-tert-butyl-4'-methoxy-dibenzoylmethane, 3.3' 5.5'-tetramethylbenzidine and sodium hyposulfite, the preparation method of the architecture comprises the following steps: (1) a storage liquid I is prepared and ready for use, and a storage liquid II is prepared and ready for use; and (2) the storage liquid I and the storage liquid II are mixed, pH value of a buffering liquid is adjusted and volume is limited. Peroxides and chromophoric substrates in the visualization reagent architecture that is provided by the invention can coexist stably without reaction for a long time, thus leading the solution architecture to be easily stored and have consistent sensitivity during measurement.
Owner:深圳生科原生物有限公司
Detection method for peroxide value of milk powder
InactiveCN102519957AGood reproducibilitySimple and fast operationMaterial analysis by observing effect on chemical indicatorFree iodineOrganosolv
The invention provides a detection method for a peroxide value of milk powder. According to the invention, fat in milk powder is extracted by using the organic solvents ethanol and ether; peroxide is produced in the oxidation process of fat and reacts with potassium iodide to produce free iodine; a standard solution of sodium hyposulfite is employed for titration, and then the content of the peroxide is calculated. The detection method is used for detection of the peroxide value of milk powder and has the advantages of good repeatability and simple operation.
Owner:AUSNUTRIA DAIRY CHINA
Method for measuring copper content in tin-silver-copper solder through iodometry
InactiveCN103776820AAccurate measurementReduce distractionsMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationPotassium thiocyanateDissolution
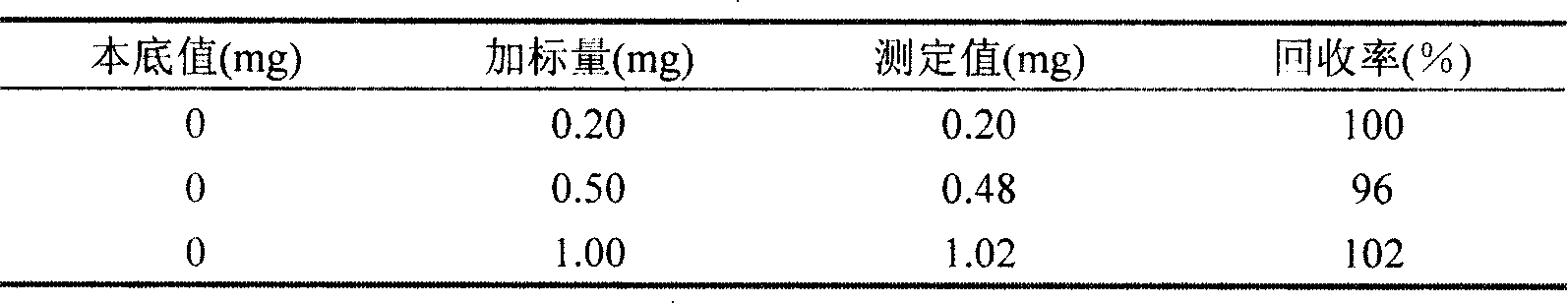
Provided is a method for measuring copper content in tin-silver-copper solder through iodometry. The method comprises the following steps: a copper standard solution is prepared and the titer of the copper standard solution is measured. A sample to be measured is weighed and added into an Erlenmeyer flask. Concentrated sulfuric acid is added and the mixture is heated for dissolution. The above solution is cooled to the room temperature, perchloric acid is added, after the sample is dissolved fully, heating is carried out until white smoke is generated, concentrated hydrochloric acid is dropwise added into the above solution in batches to remove the tin element in the solution, and the solution is subjected to concentration. The Erlenmeyer flask is taken down and cooled to the room temperature, deionized water is added, the constant volume is 100mL and the above solution is shaken up. Then an ammonium hydroxide solution is added and copper ammonia complex ions are formed. Then ammonium bifluoride is added into the solution and stirred until the blue color disappears. The above solution is cooled to the room temperature by utilization of running water. The solution is placed for half a minute, then potassium iodide is added, and immediately the solution is subjected to titration by a sodium hyposulfite standard solution until a shallow yellow color appears. Then a potassium thiocyanate solution and a starch solution are added, and the solution is subjected to titration by the sodium hyposulfite standard solution until a blue color disappears. The volume of the consumed sodium hyposulfite solution is recorded, and the content of copper in the sample is calculated.
Owner:BEIJING INST OF NONFERROUS METALS & RARE EARTH
Wheat flour benzoyl peroxide highly effective liquid phase chromatography detection method
InactiveCN101210907ANot easy to loseImprove methodComponent separationSpecial data processing applicationsBenzoic acidBenzoyl peroxide
A high-performance liquid chromatograph method for detecting benzoyl peroxide in wheat flour is carried out according to following steps of: (1) sample pre-treatment including adding absolute ethanol in a sample, infiltrating, mixing by a mixer, standing, adding potassium iodide solution to the mixer, mixing uniformly, standing, adding sodium hyposulfite solution, adding water to a predetermined volume, mixing by the mixer, standing for stratification, sucking the supernate, and filtering with a filter membrane to obtain a sample filtrate; (2) feeding the sample filtrate and a standard benzoic acid solution in a high-performance liquid chromatograph, and testing the benzoic acid concentration in the sample according to the chromatographic conditions; and (3) calculating the concentration of benzoyl peroxide in the sample according to the formula X=A*V*0.992 / (m*1000). The invention has the advantages of (1) advanced method, (2) high detection accuracy, and (3) simple and easy method.
Owner:TIANJIN FOOD RES INST
Pre-processing agent for silk broadcloth thermal transfer printing
InactiveCN101144246ARealize the perfect processSolve the problem of washing fastnessFibre treatmentDyeing processUltravioletTreatment field
The present invention belongs to the treatment field of textile, etc., in particular relates to a pretreatment agent of real silk heat transfer printing. The present invention mainly solves the technical problems existing in the prior art that the color vividness is insufficient, and the fastness cannot fully meet the need of water washing; the present invention provides the pretreatment agent of the real silk heat transfer printing with reasonable formula, greatly improves the color vividness, and has strong color intensity and fastness. The present invention has the main technical proposal that the principal component of the pretreatment agent comprises acrylic ester monomers and / or acrylic oligomer, organic siloxane, pyrrolidone, ammonia water, an ultraviolet absorbent, an emulsifier, potassium sulfate-sodium hyposulfite and deionized water.
Owner:杭州万事利丝绸礼品有限公司
Water-based printing ink emulsion for plastic foil, and preparation method thereof
InactiveCN102190990AHigh bonding strengthImprove the level ofInksEster polymer adhesivesWater basedSoftened water
The invention relates to a water-based printing ink emulsion for a plastic foil. The emulsion is prepared by the reaction among butyl methacrylate, ethyl acrylate, methyl methacrylate, diacetone-acryloamide, sodium hyposulfite, alcohol ether sodium succinate, a buffering agent, a functional auxiliary agent, ammoniacal liquor and softened water. A preparation method in the invention is also disclosed. According to the invention, the water-based printing ink emulsion enables a large bonding intensity for the plastic foil, and has the advantages of good levelability, aging resistance, no poison, no smell, safety and reliability.
Owner:上海奇想青晨新材料科技股份有限公司
Method for measuring aromatic amine in plasticine
InactiveCN102213696AAvoid matrix interferenceComponent separationPreparing sample for investigationOrganic solventGas phase
The invention discloses a method for measuring aromatic amine in plasticine, which comprises the following steps of: cutting the plasticine into small pieces, and performing ultrasonic extraction on the small plasticine pieces by using methanol, ethanol, isopropanol or acetonitrile to obtain extracting solution; removing organic solvent in the extracting solution, and adding sodium hyposulfite solution to perform a reduction reaction at the temperature of between 60 and 80 DEG C; after the reaction is finished, regulating the pH value of reaction solution to be between 10 and 12, purifying bya solid phase extraction column, and eluting to obtain eluent; and allowing the eluent to enter a gas chromatograph-mass spectrometer to measure the aromatic amine. The method for measuring the aromatic amine in the plasticine fills the blank of the technology, and the problem of matrix interference caused by other ingredients in the plasticine is solved effectively; and due to the adoption of a common detection device, the method is easy to popularize.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Kaolin for preparing heavy-oil catalytic cracking catalyst
The invention discloses kaolin for preparing a heavy-oil catalytic cracking catalyst. Preparation comprises the steps: firstly, digging a Songhua kaolin mineral aggregate, and initially separating out clay and impurities, to obtain a kaolin base material, mixing the base material and a dispersant, slurrying, then removing sand, and thus obtaining a kaolin mineral slurry initial material; transporting the kaolin mineral slurry initial material to a slurry storage tank, stirring, adding an oxidant and a sodium hyposulfite solution, and carrying out chemical bleaching, to obtain a kaolin mineral slurry concentrate; washing the kaolin mineral slurry concentrate obtained through chemical bleaching, carrying out press filtration and dewatering, and thus obtaining a filter cake; and after the filter cake is dried, crushing, and thus obtaining the finished product. The kaolin has good dispersibility and stability, can meet requirements of a catalytic cracking device on catalyst physicochemical properties, and has lower production cost; and in a gelation process, the colloid gelation time is short, the product wear index is low, and the finally-obtained catalyst has better heavy oil conversion ability and heavy metal pollution resistant ability.
Owner:茂名市茂群高岭土有限公司
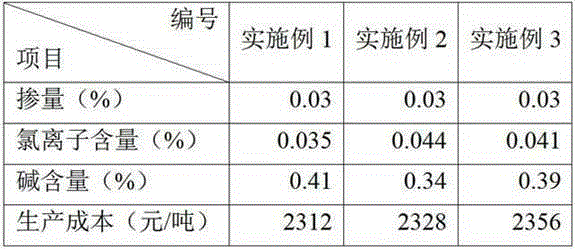
Cement grinding aid and preparation method thereof
The invention discloses a cement grinding aid and a preparation method thereof. The cement grinding aid concretely comprises, by weight, 3-7 parts of saltcake, 1-5 parts of industrial salt, 0.5-1.5 parts of sodium nitrite, 0.5-1.5 parts of sodium hyposulfite, 1-3 parts of sodium acetate, 0.5-1.5 parts of sodium sulfocyanate, 2-6 parts of triethanolamine, 0.5-1.5 parts of glycol, 0.3-0.7 parts of glycerol, 0.3-0.7 parts of polyethylene glycol, 0.5-1.5 parts of a high efficiency water reducer and 0.5-1.5 parts of a high efficiency reinforcing agent. The amounts of all materials in the cement grinding aid are controlled in order to effectively control the concentrations of chloride ions and alkalis in finished cement. Additionally, reduction of the amount of triethanolamine in the above formula greatly reduces the production cost of present cement grinding aids.
Owner:徐福明 +1
Organosilicon modified fluorine-containing polyacrylate waterproof oil resistant emulsion and preparation method thereof
The invention discloses an organosilicon modified fluorine-containing polyacrylate waterproof oil resistant emulsion and a preparation method thereof, which belongs to the emulsion production field. The emulsion is prepared by an organosilicon monomer, an emulsifier, a catalyst, a monomer A, a monomer B, sodium hyposulfite, an initiator and water with specific ratio. Compared with prior art, the organosilicon modified fluorine-containing polyacrylate waterproof oil resistant emulsion has better waterproof oil resistant performance, has simple production technology, and has good popularization and application value.
Owner:SHANDONG YUANGEN CHEM TECH RES & DEV CO LTD
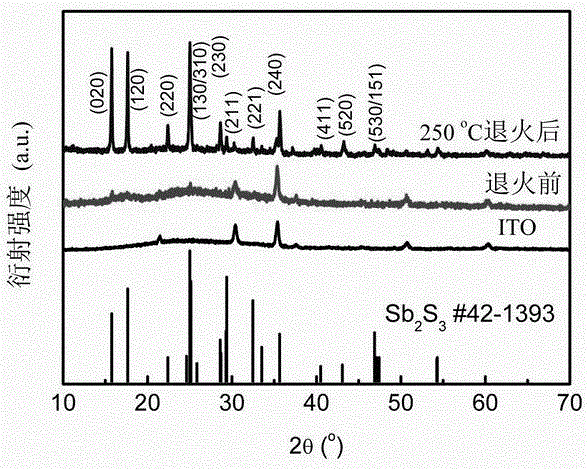
Hydrothermal preparation method of Sb2S3 semiconductor film with narrow band gap
ActiveCN105762207AHigh crystallinityFinal product manufactureSemiconductor devicesAntimony potassium tartrateMaterials science
The invention relates to a hydrothermal preparation method of a Sb2S3 semiconductor film with a narrow band gap. A solution contains antimony potassium tartrate and sodium hyposulfite with molar ratio of 10 to (9-80) and has a pH value from 4 to 4.5. After the solution is subjected to stirring, a Sb2S3 film with good adhesive force is directly prepared on an ITO glass substrate by means of a hydrothermal method. Then, the Sb2S3 film is maintained at certain constant temperature between 250 to 550 degrees centigrade under inert atmosphere so that the Sb2S3 semiconductor film with a narrow band gap is obtained. The novel plating solution in the method is friendly to environment. The Sb2S3 semiconductor film is directly prepared on the ITO glass substrate, has a narrow band gap, and is suitable to be used as an absorbing layer material of a thin-film solar cell.
Owner:BEIJING UNIV OF CHEM TECH
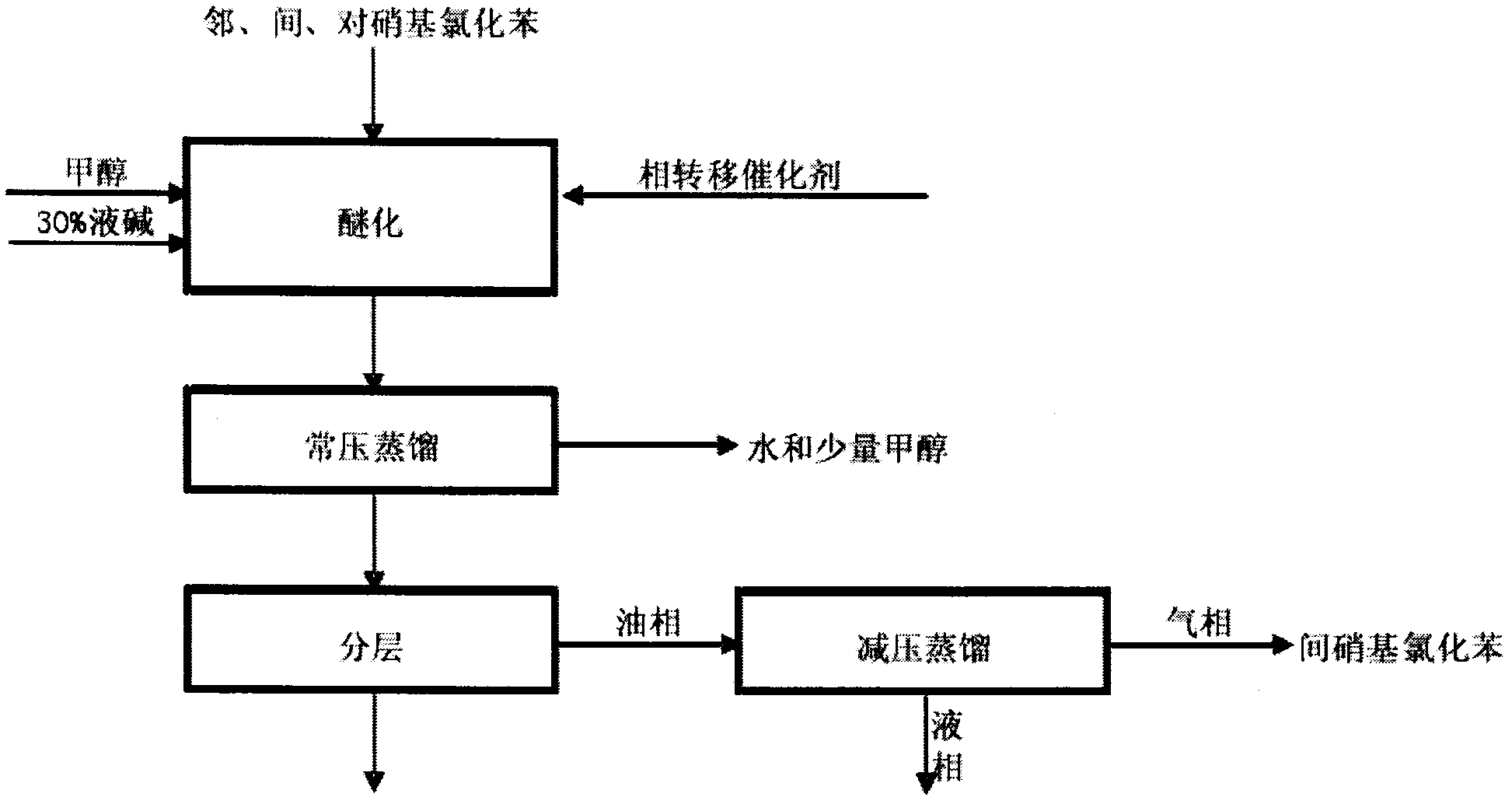
Method for preparing o-nitroanisole and p-nitroanisole from mixed nitrochlorobenzene
InactiveCN103073431AAvoid costly separationsSave energyOrganic chemistryOrganic compound preparationO-nitrochlorobenzeneChlorobenzene
The invention relates to a method for preparing o-nitroanisole and p-nitroanisole from mixed nitrochlorobenzene. According to the invention, mixed nitrochlorobenzene consisting of a product obtained after nitration of chlorobenzene, o-nitrochlorobenzene, m-nitrochlorobenzene and p-nitrochlorobenzene is used as a raw material, and in a methanol system, 30% of alkali lye and a phase-transfer catalyst are added for a reaction to prepare products of o-nitroanisole and p-nitroanisole, wherein m-nitrochlorobenzene does not participate in the reaction. The method provided by the invention overcomes the problems of great investment for rectifying tower equipment, great energy consumption in rectification, high cost, long process flow, harsh operation conditions, poor operational safety performances and generation of waste residues hardly to treat in rectification-crystallization separation of mixed nitrochlorobenzene in conventional production processes for o-nitroanisole and p-nitroanisole and overcomes the technical problems of generation of considerable alkaline waste water containing sodium hyposulfite and production of a poor-quality product in a sodium sulfide reduction process.
Owner:CHANGZHOU JIASEN CHEM
Antirust transparent cutting fluid and preparation method thereof
InactiveCN103981007AImprove rust resistanceImprove corrosion resistanceLubricant compositionPolyethylene glycolPhosphoric acid
The invention discloses an antirust transparent cutting fluid and a preparation method thereof. The antirust transparent cutting fluid comprises, by weight, 3-8 parts of triethanolamine, 1-5 parts of polyethylene glycol 2000, 0.2-0.8 parts of trisodium phosphate, 0.2-0.6 parts of polyglyceryl fatty acid ester, 0.5-1 part of sodium hyposulfite, 2-6 parts of glycerin, 1-5 parts of sodium carbonate and 80-90 parts of water. The preparation method comprises the following steps of heating water to a temperature of 60-70 DEG C, adding the above components into the water, carrying out stirring dissolution, and cooling the solution to a room temperature so that the antirust transparent cutting fluid is obtained. The antirust transparent cutting fluid has good antirust performances and anticorrosion performances, can prevent a one-grade gray cast iron single sheet from rusting at a temperature of 35+ / -2 DEG C for more than 72h, and can prevent the laminated sheets from rusting for more than 25h. A corrosion test proves that through the antirust transparent cutting fluid, at a temperature of 55+ / 2 DEG C, cast iron rusts after more than 78h and red copper rusts after more than 20h.
Owner:广州洋源化工有限公司
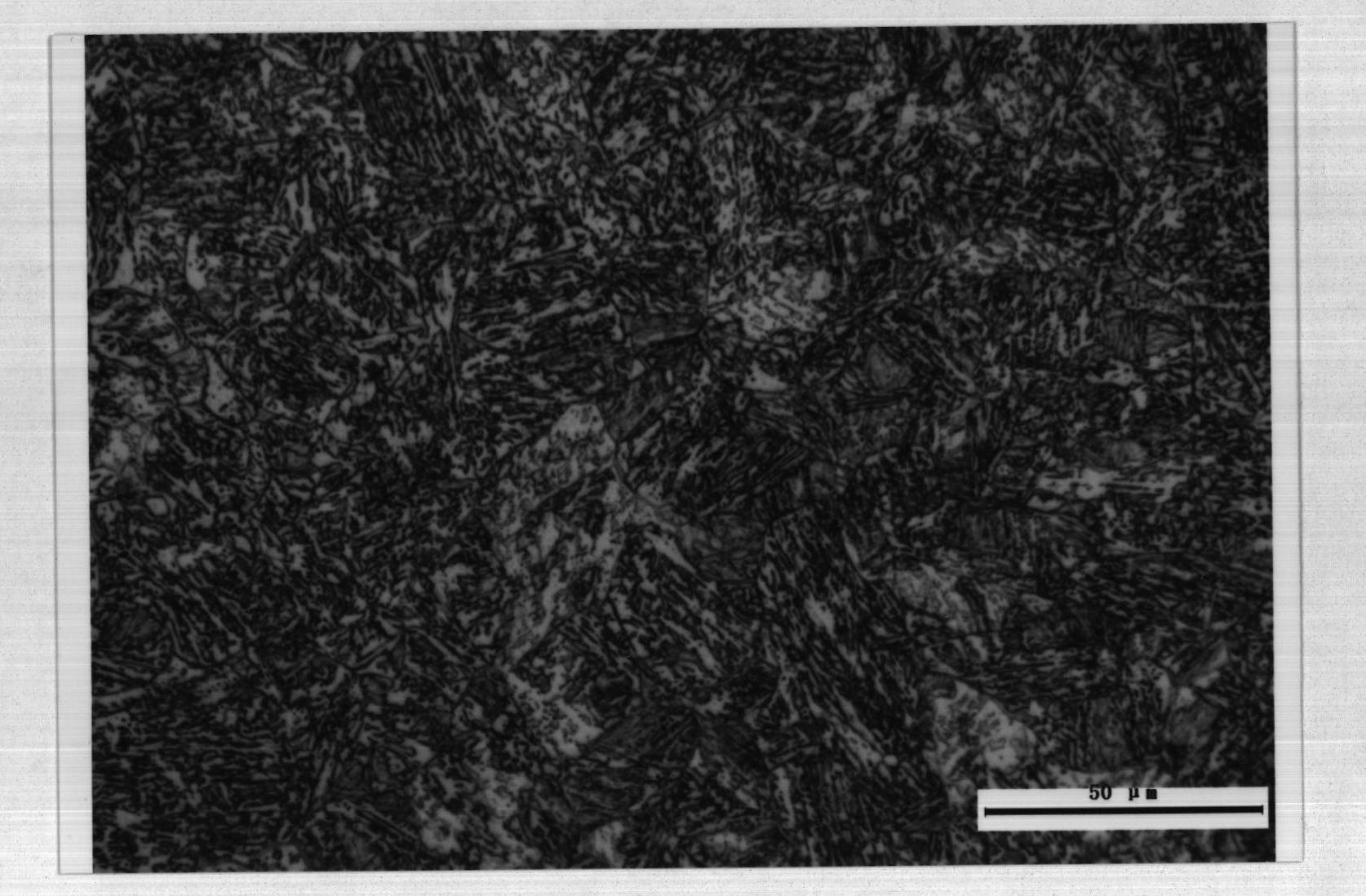
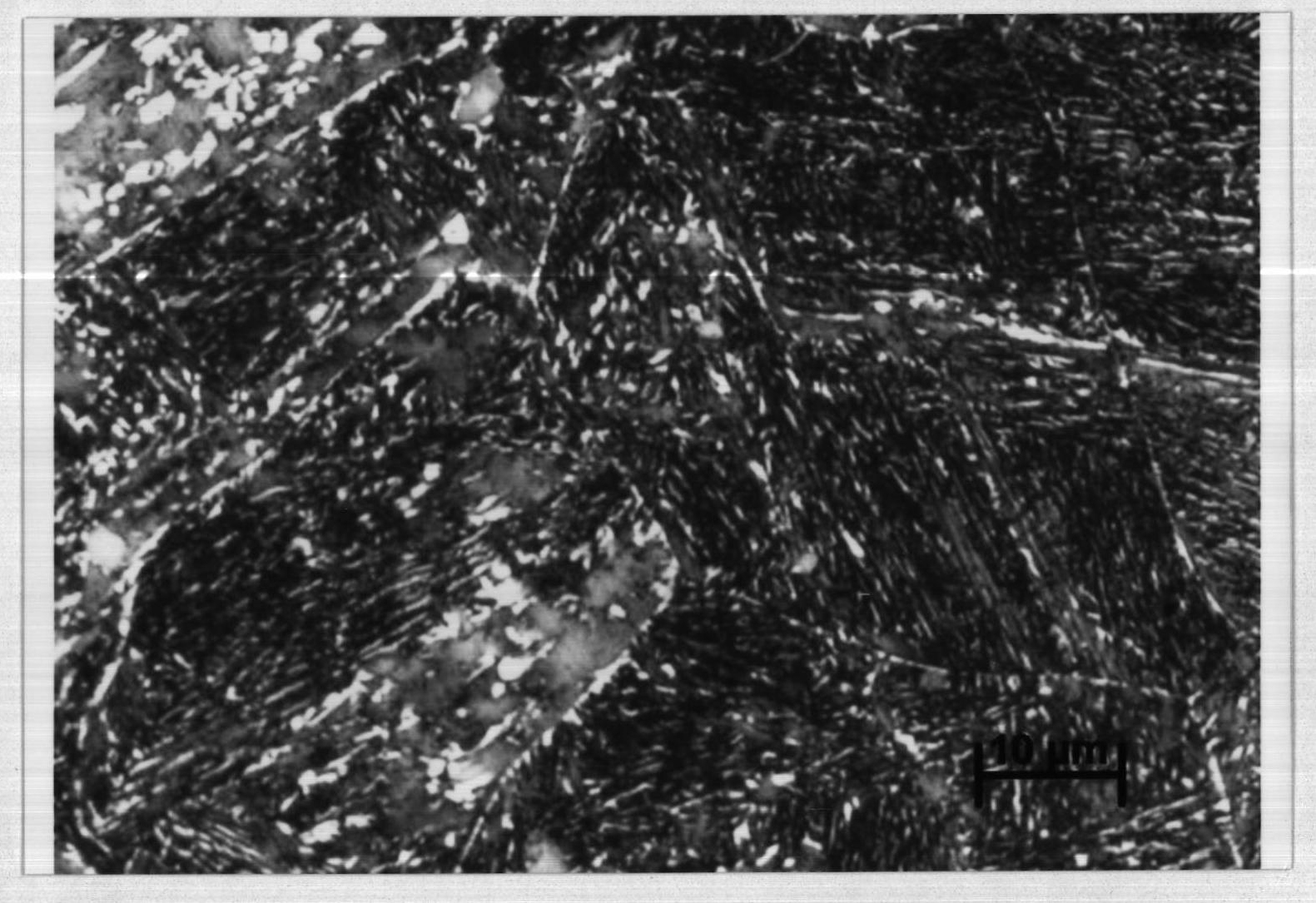
Bainite steel color metallurgical-phase dye and color display method thereof
ActiveCN101936838ANovel ideaSimple processPreparing sample for investigationSocial benefitsSodium metabisulfite
The invention relates to a Bainite steel color metallurgical-phase dye and a color display method thereof, belonging to a metallurgical-phase display dressed agent of a steel material microscopic structure, in particular to a dressed agent for a steel color metallurgical phase and a color metallurgical-phase display method. The dye is prepared from the following components: 2.5-3.5 g of sodium metabisulfite, 8-12 g of sodium hyposulfite and 100 g of water. The color metallurgical-phase display method comprises the following steps of: sampling, grinding, polishing, preparing a dye, dying to form a film, cleaning the sample, observing the color microscopic structure, and collecting electronic images and corresponding black-white metallurgical-phase images. The invention has the advantages that the Bainite steel is displayed by adopting chemical dying and the color metallurgical phase, and compared and analyzed by using the black-white metallurgical phase, which has novel conception and simple, reliable and rapid procedures; the microscopic structure of the Bainite steel can be explicitly differentiated; residential austenite, Bainite, austenite among ferrite plates and ferrite in steel are clearly differentiated; reliable guarantee is provided for the microstructure and the quantitative analysis of a steel member; the research and the development of the product are promoted and remarkable economic benefits and social benefits are obtained.
Owner:MAANSHAN IRON & STEEL CO LTD
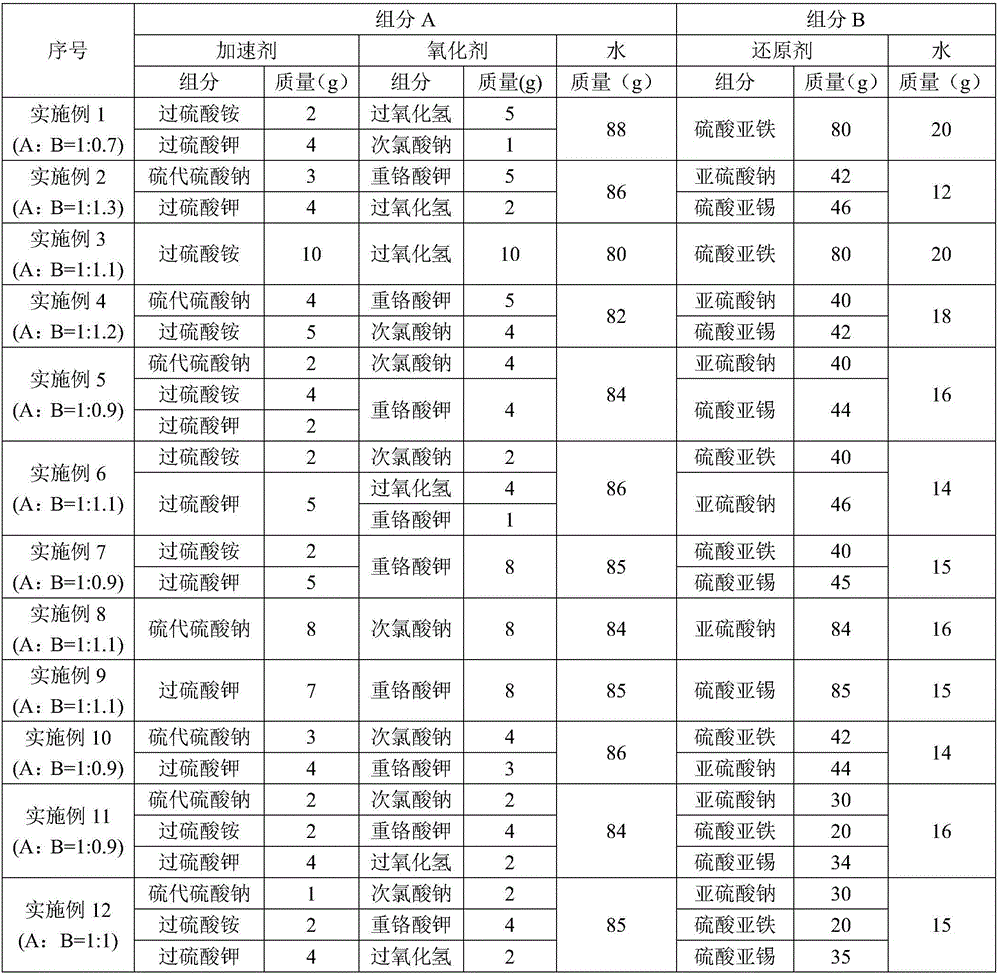
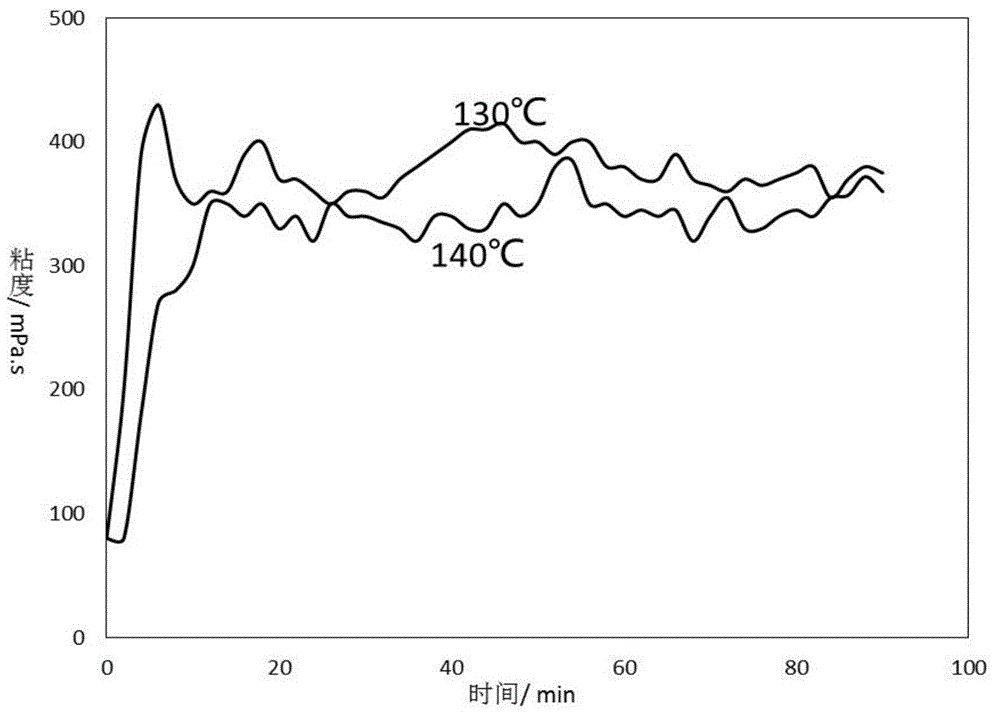
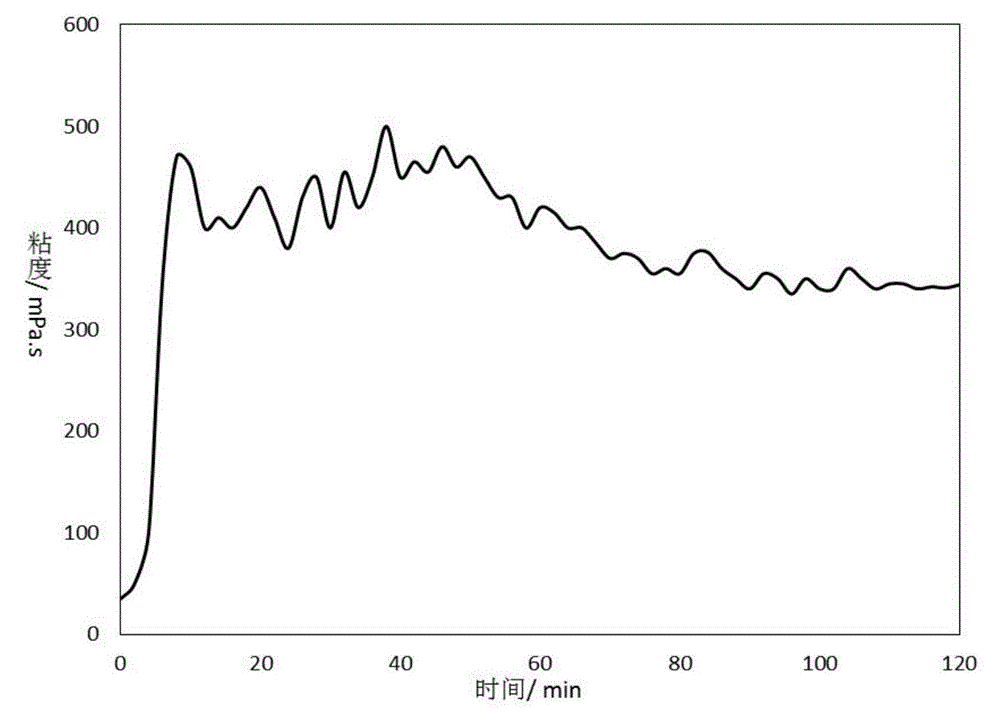
Low temperature gel breaking viscosity reducer of crosslinked guar gum backflow fracturing fluid, and preparation method thereof
InactiveCN106566517ALower the activation energy of mixed reactionsLow viscosityDrilling compositionPotassium persulfatePolymer science
The invention discloses a low temperature gel breaking viscosity reducer of a crosslinked guar gum backflow fracturing fluid, and a preparation method thereof. The low temperature gel breaking viscosity reducer is prepared through mixing a component A with a component B according to a mass ratio of 1:(0.7-1.3), and the component comprise, by mass, 6-10 parts of an accelerator, 6-10 parts of an oxidant and 80-88 parts of water, and the part sum of all the compositions of the component A is 100; the component B comprises, by mass, 80-88 parts of a reducing agent and 12-20 parts of water, and the part sum of the reducing agent and the water is 100; and the accelerant is selected from sodium hyposulfite, ammonium persulfate and potassium persulfate. The low temperature gel breaking viscosity reducer can realize rapid reaction and generation of a large amount of free radicals at a low temperature of 5-15 DEG C in order to highly-effectively destroy guar gum molecule long-chain chemical bonds and crosslinked dative bonds in the crosslinked guar gum backflow fracturing fluid, so the viscosity of the backflow fracturing fluid is effectively reduced to 2 mPa.s or below.
Owner:YANGTZE UNIVERSITY
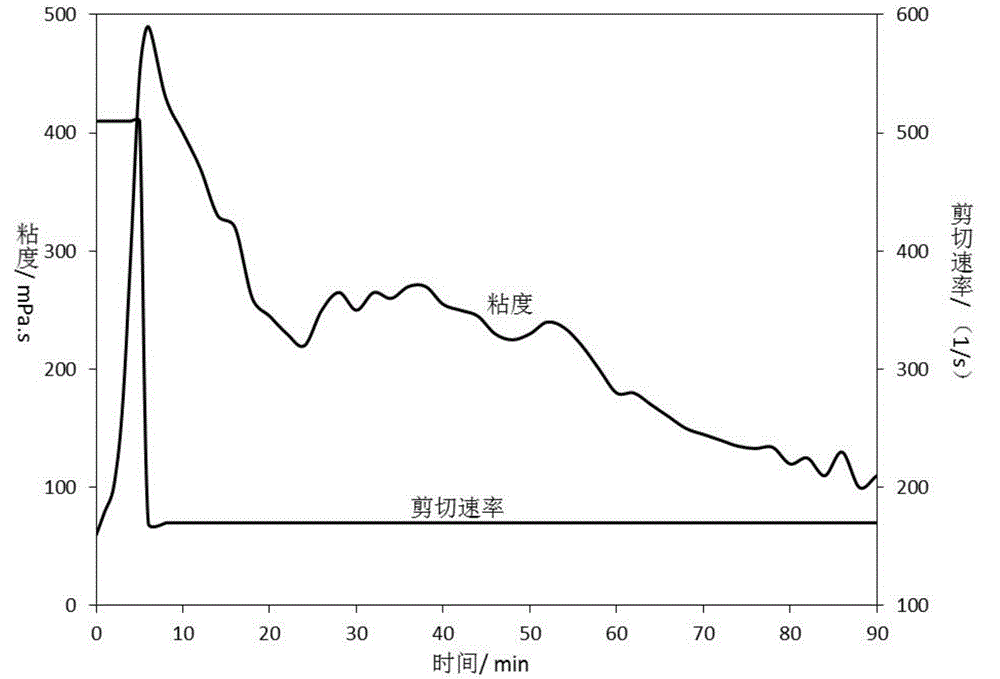
Boron cross-linking agent and boron crosslinking fracturing fluid used for high temperature deep well
ActiveCN104893705AReduce the chance of bondingControlling the speed of crosslinkingGroup 3/13 element organic compoundsDrilling compositionCross-linkFracturing fluid
The invention discloses a boron cross-linking agent and a boron crosslinking fracturing fluid used for high temperature deep well. The boron cross-linking agent is a boron-based multi-element chelate, which takes a boron-containing compound and a chelating agent as main materials and water as a solvent for reacting under certain pH value and temperature. The boron crosslinking fracturing fluid comprises the following components by mass percentage: 0.02-0.04% of boron cross-linking agent, 0.3-0.6% of thickening agent hydroxypropyl guanidine gum, 0.02-0.03% of bactericide dodecyl dimethyl benzyl chloride, 1-2% of clay stabilizing agent potassium chloride, 0.02-0.03% of high temperature stabilizing agent stabilizing agent sodium hyposulfite, 0.04-0.05% of fluorocarbon surfactant, 0.02-0.03% of PH conditioning agent sodium hydroxide and balance of water. The boron cross-linking agent can effectively control the crosslinking speed, the boron crosslinking fracturing fluid has good high temperature resistance and high shear performance, and has the advantages of low frictional resistance, easy flowback and wide market prospect.
Owner:SOUTHWEST PETROLEUM UNIV
LLDPE modified isotatic polypropylene non-woven fabric and preparation method thereof
InactiveCN103756127AHeat resistantCorrosion resistantMelt spinning methodsConjugated synthetic polymer artificial filamentsLow-density polyethyleneLinear low-density polyethylene
LLDPE modified isotatic polypropylene non-woven fabric consists of a top layer, a back layer, and a fiber cotton layer disposed between the top layer and the back layer. The top layer and the back layer are made from a non-woven fabric material, and the non-woven fabric material is prepared from the following raw materials in parts by weight by employing a melt blowing method: 100 parts of isotactic polypropylene, 2-3 parts of nanometer-level kaolin, 7-9 parts of montmorillonite, 1-2 parts of sodium hyposulfite, 2-3 parts of alumina silicate, 1-2 parts of citric acid, 1-2 parts of calcium phosphate, 12-15 parts of linear low-density polyethylene (LLDPE), 1-2 parts of an aluminate coupling agent DL-411, 12-15 parts of dioctyl terephthalate, 7-9 parts of silkworm excrement, 1-2 parts of cortex mori, 2-3 parts of ligusticum wallichii, 1-2 parts of angelica dahurica, 2-3 parts of houttuynia cordata and 4-5 parts of an auxiliary agent. The non-woven fabric provided by the invention has the characteristics of being resistant to heat, resistant to corrosion, resistant to tearing and high in strength, has aromatic smell and sterilization effect, and is capable of adsorbing harmful gases and peculiar smells by utilizing silkworm excrement for carbonization processing.
Owner:芜湖跃飞新型吸音材料股份有限公司
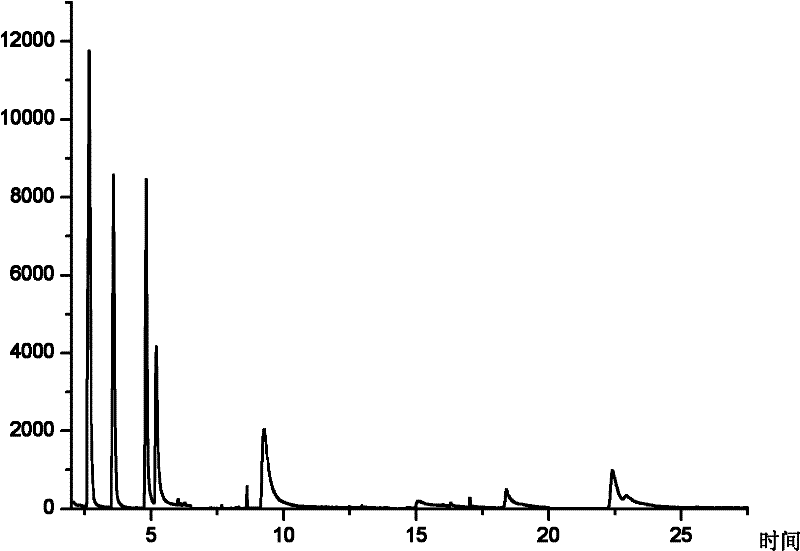
Improved detection method for sulfur dioxide
InactiveCN104155293AImprove accuracyReduce system errorMaterial analysis by observing effect on chemical indicatorDistillationDigestion
The invention provides an improved detection method for sulfur dioxide. The detection method comprises the following steps: treating a sample; placing the sample into a special digestion bottle for Kjeldahl determination, putting the digestion bottle on an azotometer, inserting the lower end of an absorption tube into a lead acetate absorption liquid in an iodine number flask, adding a hydrochloric acid solution, then immediately capping a plug, carrying out heating and distillation and carrying out blank control test during detection; adding concentrated hydrochloric acid and an iodine standard solution into a distilled fluid, placing the obtained mixture in a dark place for a reaction for 2 min, rapidly titrating an excess iodine liquid in the distilled fluid by using a sodium hyposulfite standard solution, adding a starch indicator when the distilled fluid has a light yellow color after titration and terminating titration when a blue color turns into colourless; and calculating the content of sulfur dioxide in the sample according to the consumed amount of sodium hyposulfite. According to the invention, a Kjeldahl determination apparatus is used to replace a manually-built simple distillation apparatus, so the method is simple and easily practicable, reduces system errors and improves accuracy of analysis results.
Owner:广州衡创测试技术服务有限公司
Ultra-rapid silver dye detecting method of sugarcane molecular marker PAGE gel
InactiveCN101354348AReduce usageLow costPreparing sample for investigationColor/spectral properties measurementsBiologyPolyacrylamide
The invention discloses an ultra-fast silver staining test method of cane molecule tagged PAGE gel, which comprises the following steps: a modified polyacrylamide gel with the concentration of 5 percent is used for the electrophoresis detection of SSR and AFLP tagged PCR amplified primer; the gel after the electrophoresis is placed into a dyeing liquid to be dyed; the gel is taken out from the dyeing liquid, quickly rinsed, and placed into a developing solution to be developed; the developed gel is cleaned by distilled water for two times and dried, and tape reading is implemented; the ultra-fast silver staining test method combines four traditional steps of fixing, dyeing, developing and image fixing into two simplest steps, thus simultaneously reducing rinsing frequency and time, reducing the use of a fixing liquid, an image fixing liquid, acetic acid, absolute ethyl alcohol, sodium carbonate and sodium hyposulfite, not only greatly decreasing silver staining time and reagent cost, but also ensuring high-quality silver staining effect, and compared with other traditional or improved isotopic silver staining methods, the ultra-fast silver staining test method has larger time and cost advantages.
Owner:SUGARCANE RES INST OF YUNNAN ACADEMY OF AGRI SCI
Method for detecting peroxide value of euphausia superba oil
ActiveCN106610411ANo color interference issuesImprove accuracyChemical analysis using titrationOrganic solventInterference factor
The invention provides a method for detecting the peroxide value of euphausia superba oil. The method comprises the following steps: taking a certain amount of euphausia superba oil m, dissolving the euphausia superba oil in an organic solvent, adding an excessive potassium iodide solution for a reaction, then continuously dropwise adding a sodium hyposulfite solution with the concentration being C to the obtained product and detecting the potential value of the solution till the potential value is stable, during the titration process, discretely recording the titration volume V and the potential value E of the solution, plotting a relation curve of the titration volume V and the potential value E through a second-order derivative method, searching the titration volume Vx of a potential jump point, and calculating the peroxide value of a to-be-detected sample according to the following formula: P'=(Vx-V0)*C / 2m, wherein V0 is the titration volume of a potential jump point detected in a blank sample according to the method above. According to the method, problems that the detection accuracy of an iodometric method is low, and many interference factors result from a solvent, the temperature, and a pH value are solved.
Owner:SHENZHEN INST OF ADVANCED TECH
Slurry composition for transportation and fresh-keeping processing of seedling distance-transferring bare roots and processing method thereof
InactiveCN102657151AEasy to handlePrevents the danger of excessive dehydrationDead plant preservationSeedlingSodium sulfate
The invention relates to a slurry composition for transportation and fresh-keeping processing of seedling distance-transferring bare roots and a processing method thereof. The slurry composition comprises the following raw materials of: by weight, 0.5-2% of a plant antistaling agent, 1-5% of vitality nutrient, 40-70% of neutral clay soil, and the balance being water. The contents of the plant antistaling agent are as follows: silver nitrate: sodium hyposulfite: sugar: water is 1:11:400:2000. The processing method comprises the following steps of: watering roots and spraying leaves by the use of a chemical antistaling agent every 5-7 days before taking out the seedling (Chinese rose) bare roots; using the vitality nutrient, and respectively using a root watering liquid to water roots and spraying leaves every 7-15 days; taking out the Chinese rose which has undergone root watering and leaf spraying processing to make the roots bare; coating the roots with multilayers of slurry, putting into a plastic bag and wrapping by the use of a sunshade net, and tidily moving the processed Chinese rose onto a transport means and transporting to a destination. The survival rate of the processed seedling can reach more than 98.6%. The processing method is simple to operate. By the processing method, the survival rate of the long-distance transportation seedling is raised and the transportation cost is effectively saved. The invention is of great significance for long-distance transportation of seedlings.
Owner:TIANJIN UNIV
Rapid analysis method of lead in gold mud
ActiveCN105044097AFill vacancyEliminate distractionsMaterial analysis by observing effect on chemical indicatorSodium acetateSodium acetrizoate
The invention relates to a rapid analysis method of lead in gold mud. The method comprises the following steps: processing a sample by nitric acid, aqua regia, sulfuric acid, and a mixed acid of nitric acid and sulfuric acid in sequence, after the acid is smoked and evaporated, adding diluted sulfuric acid, boiling to dissolve the salts, adding excess sodium hyposulfite to reduce gold to zero valent and precipitate silver; allowing the system to stand still to carry out aging, filtering, washing the precipitate by diluted sulfuric acid and water; dissolving the precipitate by an acetic acid-sodium acetate buffer solution, filtering, recovering gold and silver, adding a xylenol orange indicator into the filtrate, dropwise adding EDTA until the color of the solution changes from purple-red to light yellow, wherein the color change shows that the titration end point is reached, and finally calculating the lead content. In the provided method, sodium hyposulfite is used to reduce gold and precipitate silver so as to effectively separated gold and silver from lead, thus the interference of gold and silver on the end point is avoided, moreover, the operation is largely simplified, and precise and stable analysis results can be obtained by the provided method.
Owner:山东黄金冶炼有限公司
Method for determining content of sulfite in wet desulphurization slurry
ActiveCN103149201AImprove accuracyImprove reliabilityMaterial analysis by observing effect on chemical indicatorSodium acetateSlurry
The invention relates to a method for determining the content of sulfite in a wet desulphurization slurry. The method comprises the following determination steps: 1, adding 50ml of an acetic acid-sodium acetate buffer solution having a pH value of 4.0-5.7 and 10.00ml of a 0.1mol / L iodine standard solution to a stopper possessed measuring flask; 2, adding a tested sample filtrate until the iodine solution becomes flavescent or tannish, and carrying to a laboratory under a dark condition; 3, adding 1ml of a 1% starch solution to make the tested sample become blue; 4, titrating the sample solution with a 0.1mol / L sodium hyposulfite standard solution until the blue color disappears, and recording the consumption amount of the sodium hyposulfite standard solution; carrying out a blank experiment; and 6, calculating to obtain the content of sulfite. The method which allows the buffer solution to be added in the sampling process has the advantages of avoiding of the oxidation in the sampling process, prevention of the interference of components comprising S2O3<2-> and the like in the test process, increase of the interference resistance of the slurry in the sampling and testing process, and improvement of the accuracy and the reliability of the test result.
Owner:STATE GRID HEBEI ELECTRIC POWER RES INST +2
Acidolysis oxidation conversion method for extracting vanadium from stone coal
InactiveCN101210287AMild reaction conditionsReduce consumptionProcess efficiency improvementLiquid ratioVanadyl sulfate
A method for extracting vanadium in stone coal by acidolysis, oxidation and conversion comprises the following steps: (1) pulverizing stone coal and grinding to powder of 60 to 120 meshes; (2) adding water and concentrated sulfuric acid according to a weight ratio of stone coal powder to concentrated sulfuric acid to water of 100:(15-25):(6-10), stirring uniformly and standing for 16 to 60 days in an open manner; (3) adding water according to a solid-to-liquid ratio of 1:(1-5) and stirring for 1 to 4 h; (4) adding reducer sodium hyposulfite according to the content of Fe contained in the solution to reduce Fe in the solution if Fe(II) or / and Fe(III) is / are contained in the solution; and (5) adjusting pH value to 2 to 3, heating to 30 to 60 DEG C, and filtering to obtain a blue filtrate containing vanadyl sulfate. The invention has the advantages of simple process, low requirement for equipment, high vanadium leaching efficiency (not less than 88%), less reagent consumption, less pollution, etc.
Owner:谢桂文
Restoration agent for heavy-metal Hg polluted soil, and preparation method and application method thereof
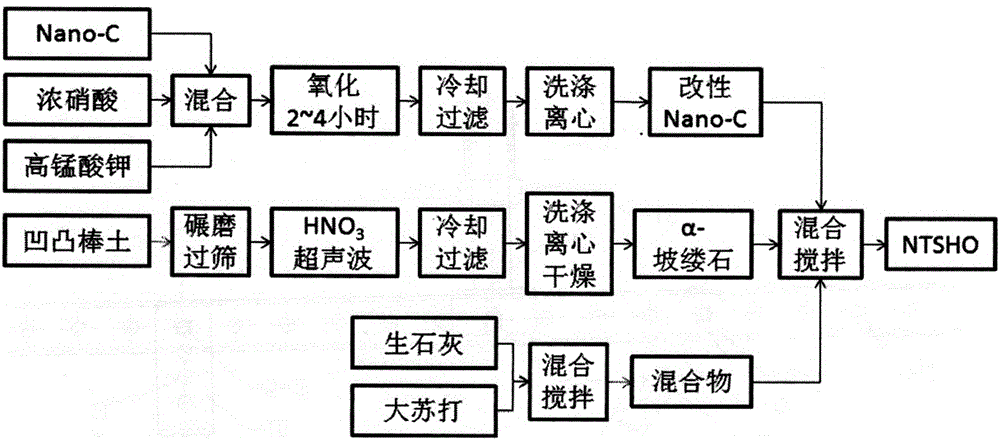
ActiveCN105038805ALow costSimple processContaminated soil reclamationOrganic fertilisersSoil sciencePalygorskite
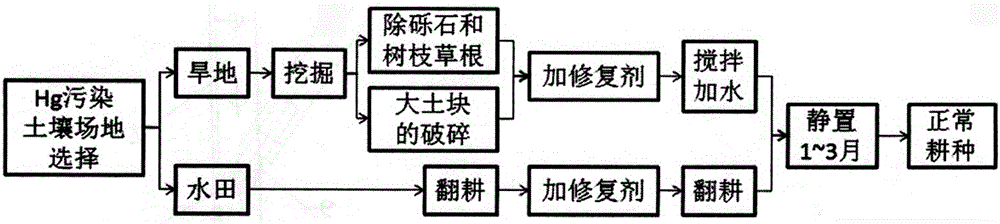
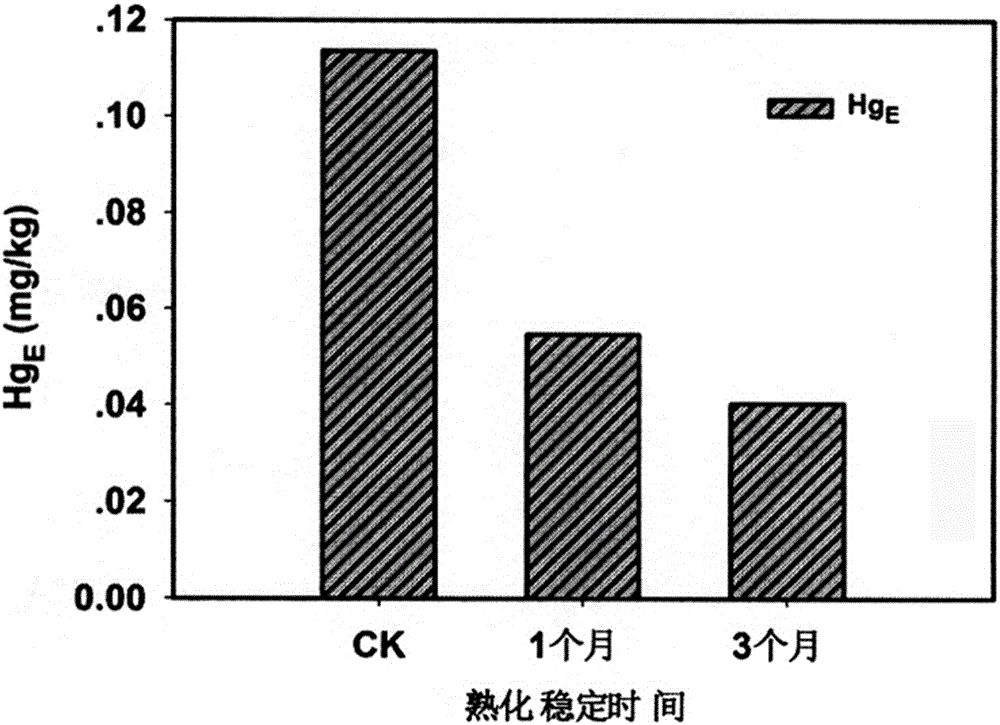
The invention discloses a restoration agent for heavy-metal Hg polluted soil, and a preparation method and application method thereof. The restoration agent is composed of the following components in parts by weight: 0.1-15 parts of modified NanoC, 5-40 parts of alpha-paligorskite and 40-70 parts of quicklime+sodium hyposulfite mixture. The NanoC is oxidized and modified by acidic potassium permanganate; and the alpha-paligorskite is prepared by modifying natural attapulgite by HNO3 solution immersion and high-temperature heat treatment. The application method of the restoration agent is as follows: the restoration agent accounts for 0.1-5.0% of polluted soil; as for the polluted soil with the THg concentration of less than 20 ppm, the addition amount is 0.3-0.5%; and the restoration agent is sufficiently and uniformly mixed with the soil in a plowing or stirring mode, and the water content of 10-30% relative to the total weight of the soil is kept for 1-3 months.
Owner:INST OF MINERAL RESOURCES CHINESE ACAD OF GEOLOGICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com