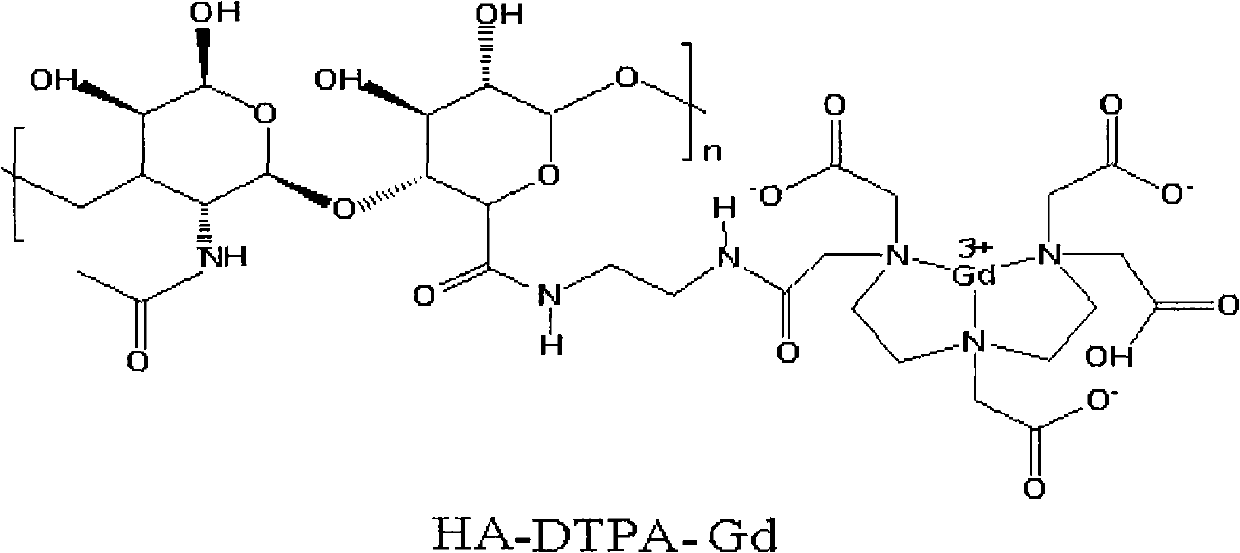
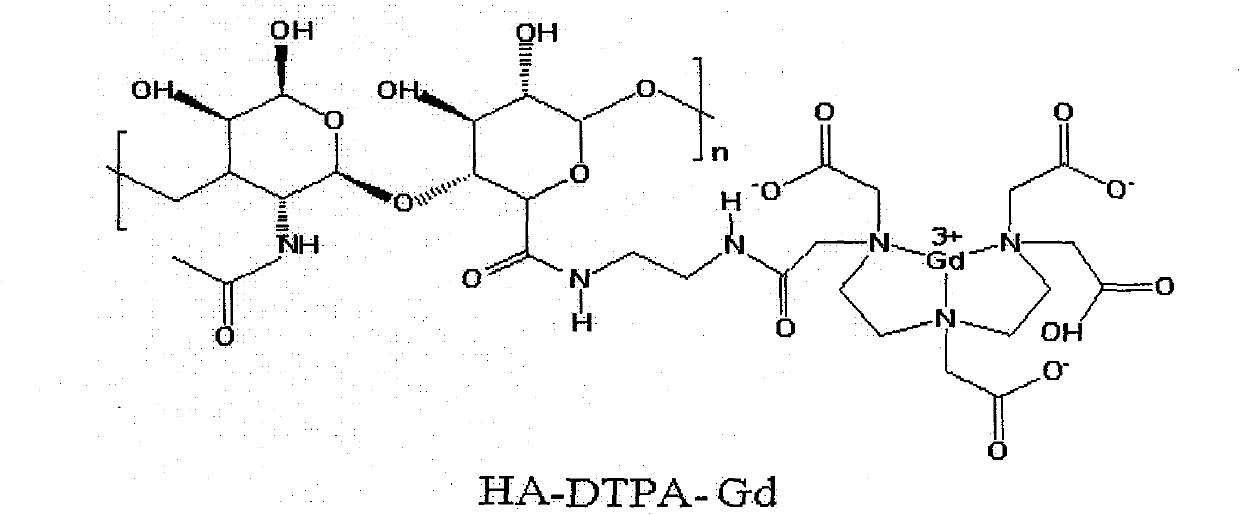
Gadolinium-containing macromolecular contrast agent for specific imaging of lymphatic system and preparation method thereof
A lymphatic system and contrast agent technology, applied in the direction of MRI/MRI contrast agent, etc., can solve the problems of lack of basement membrane and high permeability, achieve high grafting rate, prevent interference, and eliminate the interference of capillaries Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Fully dissolve 400mg of hyaluronic acid (HA) in 50ml of 2-(N-morpholine)ethanesulfonic acid (MES) buffer solution (0.1mol / L, pH5.0), and slowly add 200mg1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC), 120mg N-hydroxysuccinimide (NHS) and stirred at room temperature for 12 hours, thereafter in the reaction solution 1200 mg of ethylenediamine (EDA) was added and stirring was continued for 12 hours at room temperature. Then add the reaction solution into a dialysis bag with a molecular weight cut-off of 30,000, dialyze in deionized water for 7 days, and then freeze-dry, then fully dissolve the obtained product in 50ml of deionized water to obtain solution a; take 1000mg of diethylenetriaminepenta Acetic acid (DTPA) was dissolved in 10ml dimethyl sulfoxide (DMSO), fully stirred under nitrogen flow until dissolved, then added 1760mg triethylamine (TEA), 514mg N, N'-dicyclohexylcarbodiimide ( DCC), 288mg N-hydroxysuccinimide (NHS), stirred at room temperature...
Embodiment 2
[0030] Example 2: Fully dissolve 50mg of hyaluronic acid (HA) in 10ml of phosphate (PBS) buffer solution (0.02mol / L, pH6.0), slowly add 25mg of 1-(3-dimethylamino Propyl)-3-ethylcarbodiimide (EDC), 10mgN-hydroxysuccinimide (NHS) and stirred at room temperature for 12 hours, thereafter in the reaction solution was added 10mg ethylenediamine (EDA), Stirring was continued for 12 hours at room temperature. Then add the reaction solution into a dialysis bag with a molecular weight cut-off of 30,000, dialyze in deionized water for 7 days, and then freeze-dry, then fully dissolve the obtained product in 10ml of deionized water to obtain solution a; take 100mg of diethylenetriaminepenta Dissolve acetic acid (DTPA) in 1ml of acetonitrile (ACN), stir well under nitrogen flow until dissolved, then add 200mg of triethylamine (TEA), 50mg of CMC, 25mg of N-hydroxysuccinimide (NHS), room temperature Stir at low temperature for 12 hours to obtain solution b; slowly drop solution a into solut...
Embodiment 3
[0031] Example 3: Fully dissolve 1000mg of hyaluronic acid (HA) in 1000ml of phosphate (PBS) buffer solution (1mol / L, pH6.0), slowly add 1000mg of 1-cyclohexyl-3-(2-methanol) under stirring Pheninoethyl)-carbodiimide p-toluene methanesulfonic acid (CMC), 1000mg N-hydroxysuccinimide (NHS) and stirred at room temperature for 24 hours, after which 3000mg ethylenediamine was added in the reaction solution (EDA), stirring was continued at room temperature for 24 hours. Then add the reaction solution into a dialysis bag with a molecular weight cut-off of 30,000, dialyze in deionized water for 14 days, and then freeze-dry, then fully dissolve the obtained product in 1000ml of deionized water to obtain solution a; Acetic acid (DTPA) was dissolved in 200ml tetrahydrofurfuran (THF), fully stirred under the atmosphere of nitrogen flow until dissolved, then added 5000mg triethylamine (TEA), 2000mg N,N'-diisopropylcarbodiethylene Amine (DIC), 4000 mg N-hydroxysuccinimide (NHS), stirred at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com