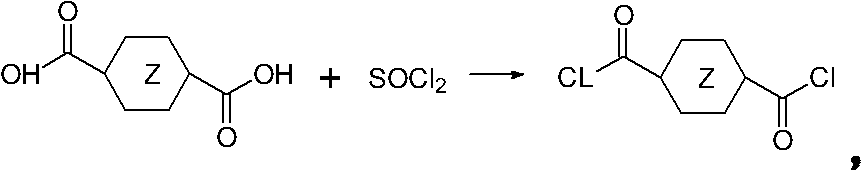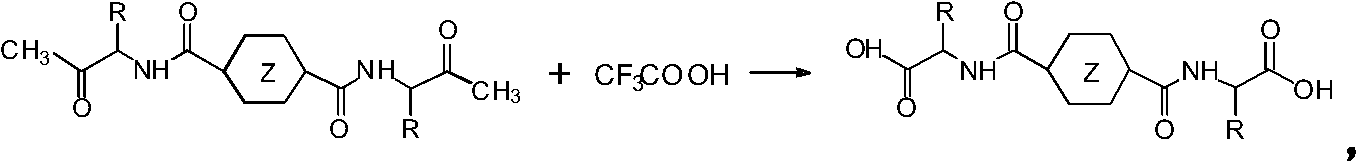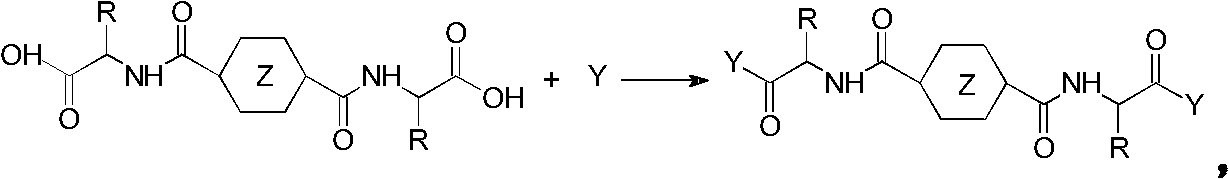Method for preparing hydrogel material based on 1,4-cyclohexane/phthalic acid
A technology of phthalic acid and cyclohexane, which is applied in the field of preparation of hydrogel materials, can solve the problems of difficult preparation of hydrogels, harsh and complex synthesis conditions, etc., and achieve good biocompatibility and rapid gelation , good water absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037] SDiAcNHPhCOCH 2 CH 2 Synthesis of OH
[0038] a) Synthesis of 1,4-cyclohexanedicarboxylic acid chloride SDiAcCl
[0039] 1,4-cyclohexanedicarboxylic acid (4.0 g, 20 mmol) and SOCL 2 (12ml) mixed in a flask. Heated to reflux for 6 hours to obtain a yellow transparent solution. Rotary evaporation to remove residual SOCl 2 , to obtain an oil, and then placed in the refrigerator (-4 ° C) to obtain a pale yellow solid sample.
[0040] b) Synthesis of SDiAcNHPhCOMe
[0041]
[0042] At 0 °C, SDiAcCl (4 mmol) in anhydrous CH 2 Cl 2 solution was added to HCl.NH 2 -L-Ph-OMe(9mmol) and Et 3 N (3ml) in 30ml anhydrous CH 2 Cl 2 solution. The reaction was allowed to warm slowly to room temperature and stirred for 6 hours. The resulting precipitate was collected by vacuum filtration and washed with ethanol. Yield (46%). 1 H-NMR (DMSO): δ1.32(m.4H); δ1.78(m.4H); δ2.17(s.2H); δ2.71(d.2H); δ2.88(d.2H ); δ3.62 (s.6H); δ4.41 (s.2H); δ7.22 (m.Ph, 10H), δ7.96 (s.2H).
...
example 2
[0050] Synthesis of SDiAcNHPhCOhistamine
[0051]
[0052] 1-Hydroxybenzotriazole monohydrate (HOBT) (Mw 135, 1.32 mmol, 2.2 times) and SDiAcNHPhCOOH (0.6 mmol) were dissolved in 5 ml of DMSO solution at room temperature. Dissolve 1,1'-carbonyldiimidazole CDI (Mw 162, 1.32 mmol, 2.2 times) in 1 ml DMSO, and then add dropwise to the above solution. Then stir for 2 hours.
[0053] Histamine (1.32 mmol, 2.2 times) was dissolved in 1 ml DMSO, then added dropwise to the above mixed solution and stirred at room temperature for 24 hours. After the reaction was completed, the reaction mixture was poured into 20 ml of water, filtered to obtain the precipitated part, and then washed with water and methanol respectively. The precipitated fraction was collected and dried in vacuo. A pale yellow solid powder was obtained with a yield of 75%. 1 H-NMR (DMSO): δ1.36(m.4H); δ1.75(m.4H); δ2.13(s.2H); δ2.75(d.2H); δ2.68(d.2H ); δ4.54 (s.2H); δ6.89 (s.2H); δ7.12 (m.Ph, 10H); δ7.44 (s.2H);...
example 3
[0055] Synthesis of SDiAcNHSCOLysine
[0056] a) Synthesis of SDiAcNHSCOMe
[0057]
[0058] At 0 °C, SDiAcCl (4 mmol) in anhydrous CH 2 Cl 2 solution was added to HCl.NH 2 -L-Methine-OMe (9mmol) and Et 3 N (3ml) in 30ml anhydrous CH 2 Cl 2 solution. The reaction was allowed to warm slowly to room temperature and stirred for 6 hours. The resulting precipitate was collected by vacuum filtration and washed with ethanol. Yield (80%). 1 H-NMR (DMSO): δ1.37(m.4H); δ1.72(m.4H); δ2.03(m, 10H, SCH 3 +CH 2 ); δ2.61(m, 4H, SCH 2 );δ2.23(s.4H);δ2.72(d.2H);δ2.78(d.2H);δ3.62(s.6H);δ4.56(s.2H)δ8.10( s.2H).
[0059] b) Synthesis of SDiAcNHSCOOH
[0060]
[0061] SDiAcNHSCOMe (0.6 mmol) was added to MeOH (10 ml) and 2M NaOH at 0°C. The reaction mixture was slowly warmed to room temperature and stirred for 4 hours. Dilute with water and adjust the pH of the solution to less than 4 with 6M HCl. The resulting precipitate is collected and dried in vacuo. Yield 81%.
[0062...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com