Gel medicine sustained release preparation based on hydrophobically modified gemcitabine derivative as well as preparation method and application of gel medicine sustained release preparation
A technology of gemcitabine and hydrophobic modification, applied in the field of drug preparation, can solve the problems of reducing the bioavailability of drugs, unable to obtain anti-tumor effect, etc., and achieve the effects of improving in vivo stability, simple and practical preparation process, and convenient practical operation and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
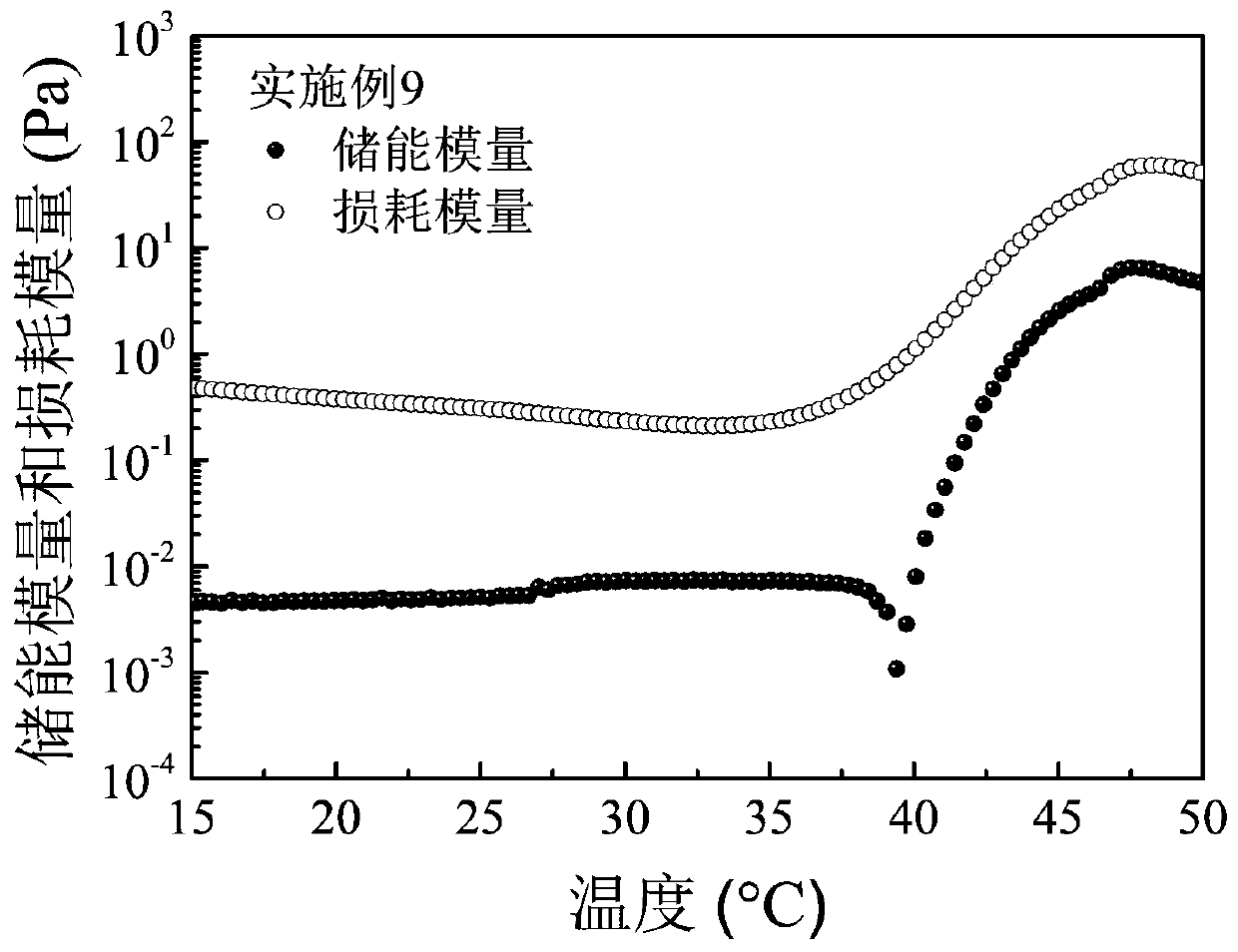
Embodiment 1
[0055] Add dihydroxypolyethylene glycol (PEG1500) into a 250 mL three-necked flask, and remove water under vacuum at 120° C. for 3 hours. Cool to 80°C with argon, add lactide (LA), glycolide (GA) and stannous octoate (containing a small amount of toluene), and vacuum at 120°C for 30 minutes. Argon was blown, and the temperature was raised to 150° C. to react for 12 hours. After the reaction was completed, vacuum filtration was performed for 2 hours to remove unreacted monomers and low-boiling products in the system. The product was poured into deionized water at 80°C while it was hot, washed three times repeatedly, and freeze-dried to obtain the BAB type triblock polymer PLGA-PEG-PLGA with a yield of about 81%. The number-average and weight-average molecular weight (M n ,M w ) are 5280 and 6340 respectively, molecular weight distribution coefficient (M n / M w , ) is 1.20, and its polymer water system does not have heat-induced gelation performance.
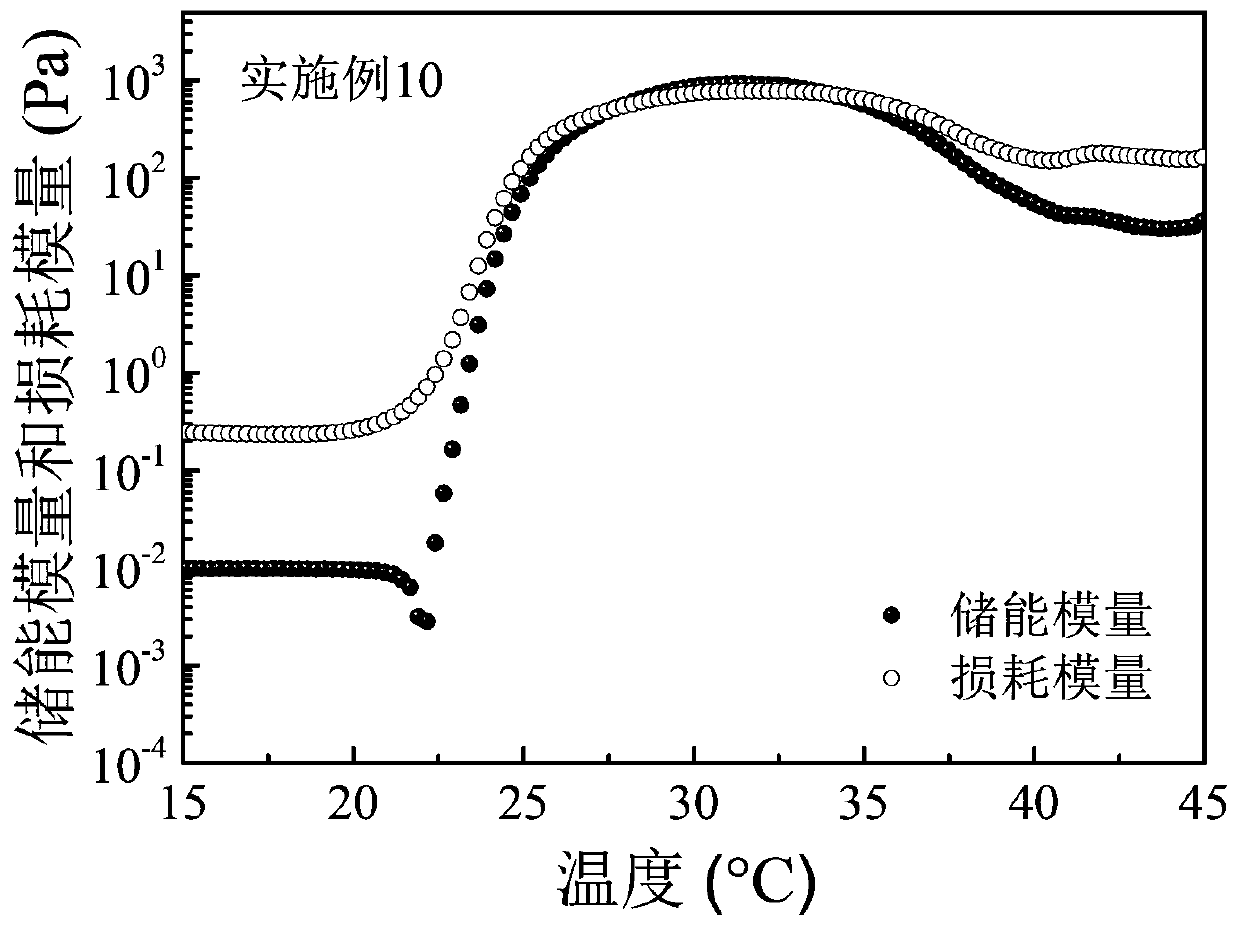
Embodiment 2
[0057] Add dihydroxypolyethylene glycol (PEG1000) into a 250 mL three-necked flask, and remove water under vacuum at 120° C. for 3 hours. Cool to 80°C with argon, add lactide (LA), glycolide (GA) and stannous octoate (containing a small amount of toluene), and vacuum at 120°C for 30 minutes. Argon was blown, and the temperature was raised to 150° C. to react for 12 hours. After the reaction was completed, vacuum filtration was performed for 2 hours to remove unreacted monomers and low-boiling products in the system. The product was poured into deionized water at 80°C while it was hot, washed three times repeatedly, and freeze-dried to obtain the BAB type triblock polymer PLGA-PEG-PLGA with a yield of about 87%. The number-average and weight-average molecular weight (M n ,M w ) are 5210 and 6760 respectively, molecular weight distribution coefficient (M n / M w , ) is 1.30, and its polymer water system does not have heat-induced gelation performance.
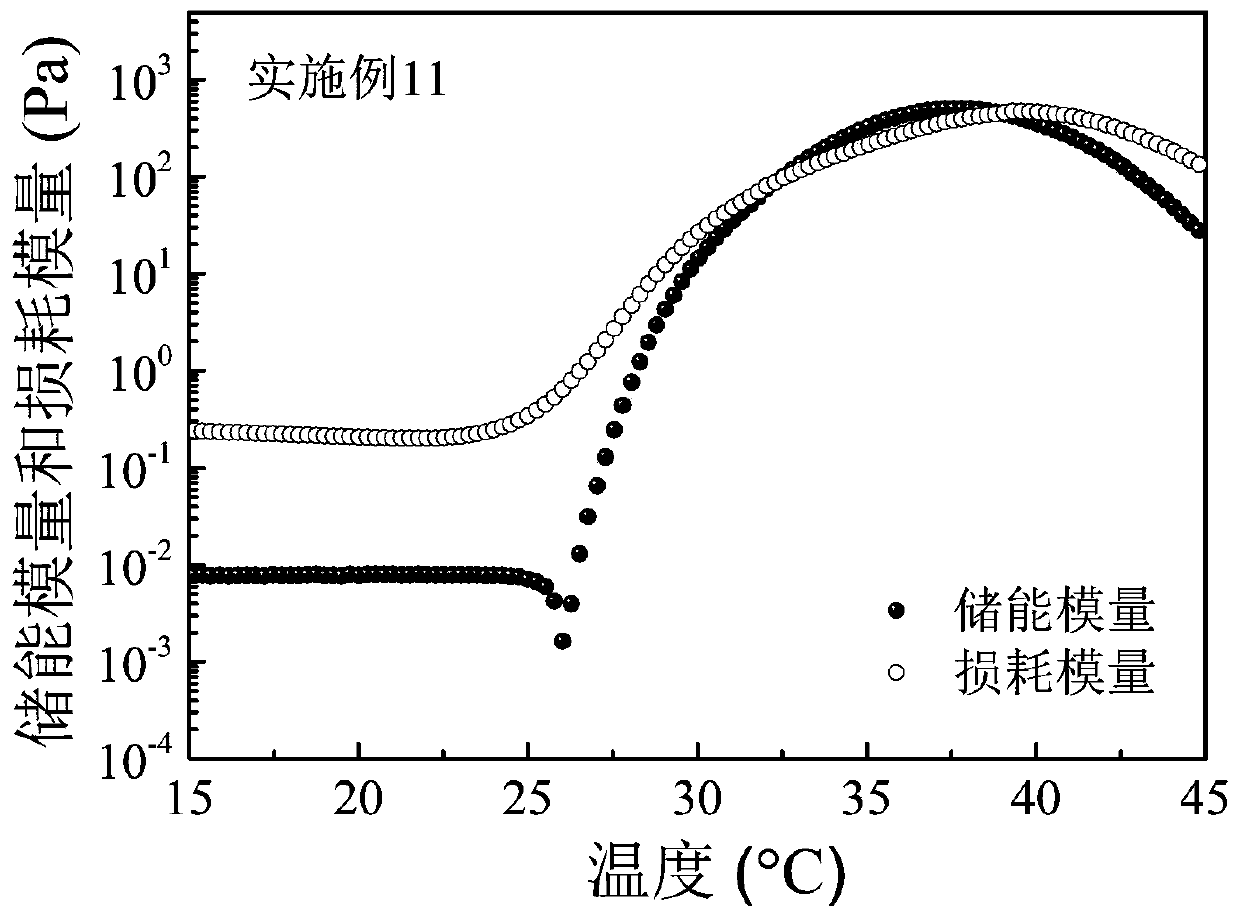
Embodiment 3
[0059] Add monomethoxypolyethylene glycol (mPEG550) into a 250 mL three-necked flask, and remove water under vacuum at 120° C. for 3 hours. Cool to 80°C with argon, add lactide, glycolide and stannous octoate (containing a small amount of toluene), and vacuum at 120°C for 30 minutes. Argon was blown, and the temperature was raised to 150° C. to react for 12 hours. After the reaction, the initial product was dissolved in dichloromethane solution, precipitated with ether, and vacuum-dried for 48 hours to obtain the AB-type diblock polymer mPEG-PLGA with a yield of about 80%. By gel permeation chromatography (GPC, polystyrene is a standard sample), the number average and weight average molecular weight (M n ,M w ) are 4680 and 6100 respectively, molecular weight distribution coefficient (M n / M w , ) is 1.30, and its polymer water system does not have heat-induced gelation performance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com