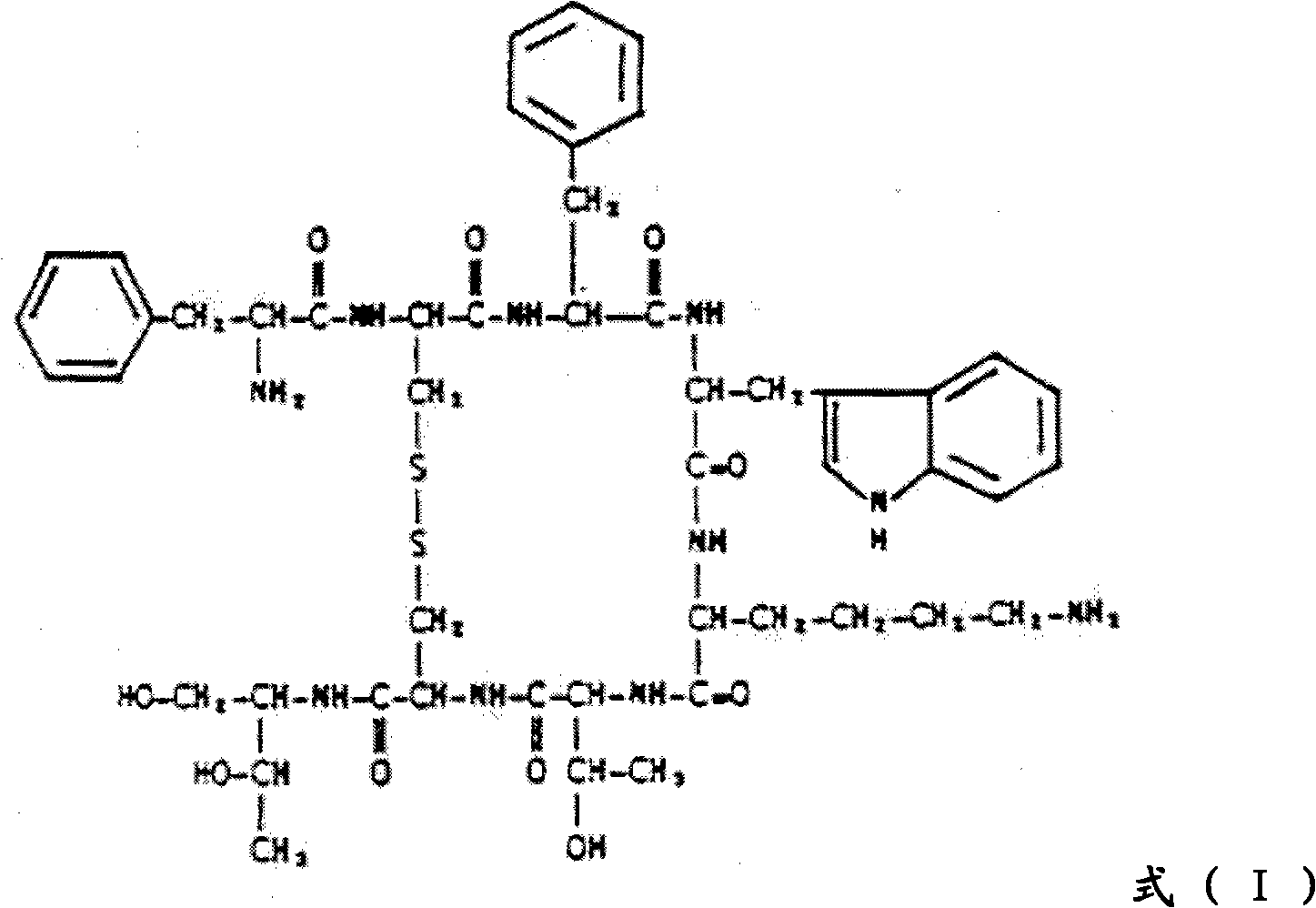
Preparation method of octreotide
A technology of octreotide and crude peptide, which is applied in the field of solid-phase synthesis of octreotide, can solve the problems of carcinogenic by-products and low yield of octreotide, and achieve the effect of increasing product yield and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] The abbreviations used in the present invention have the following meanings:
[0029] Boc: tert-butoxycarbonyl; tBu: tert-butyl; BOP: benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate; Bzl: benzyl; 2-CTC: 2 -Chlorotrityl; DCM: dichloromethane; DIC: N,N-diisopropylcarbodiimide; DIPEA: N,N-diisopropylethylamine; DMF: N,N-dimethyl Formamide; EDT: 1,2-ethanedithiol; Fmoc: 9-fluorenylmethoxycarbonyl; HOBt: 1-hydroxybenzotriazole; TFA: trifluoroacetic acid; Trt: trityl; Z: benzyl Oxycarbonyl;
[0030] Fmoc-AA-OH: amino acid protected by N-fluorenylmethoxycarbonyl;
[0031] Fmoc-Cys(Trt)-OH: N-fluorenylmethoxycarbonyl-S-tritylcysteine;
[0032] Fmoc-Lys(Boc)-OH: N-α-fluorenylmethoxycarbonyl-N-ε-tert-butoxycarbonyllysine;
[0033] Fmoc-Phe-OH: N-fluorenylmethoxycarbonyl-phenylalanine;
[0034] Fmoc-D-Phe-OH: N-fluorenylmethoxycarbonyl-D-phenylalanine;
[0035] Fmoc-Thr(tBu)-OL: N-fluorenylmethoxycarbonyl-O-tert-butylthreoninol;
[0036] Fmoc-Thr(tBu)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com