Method for preparing CdS-Bi2S3 composite nanocrystalline by utilizing partial cation exchange reaction
A cation exchange, cds-bi2s3 technology, applied in nanotechnology, nanotechnology, nanostructure manufacturing, etc., can solve the problems of high cost, complex nanocrystal synthesis, increase the complexity and cost of the synthesis process, and achieve convenient operation, experimental The effect of low equipment requirements and simple experimental process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
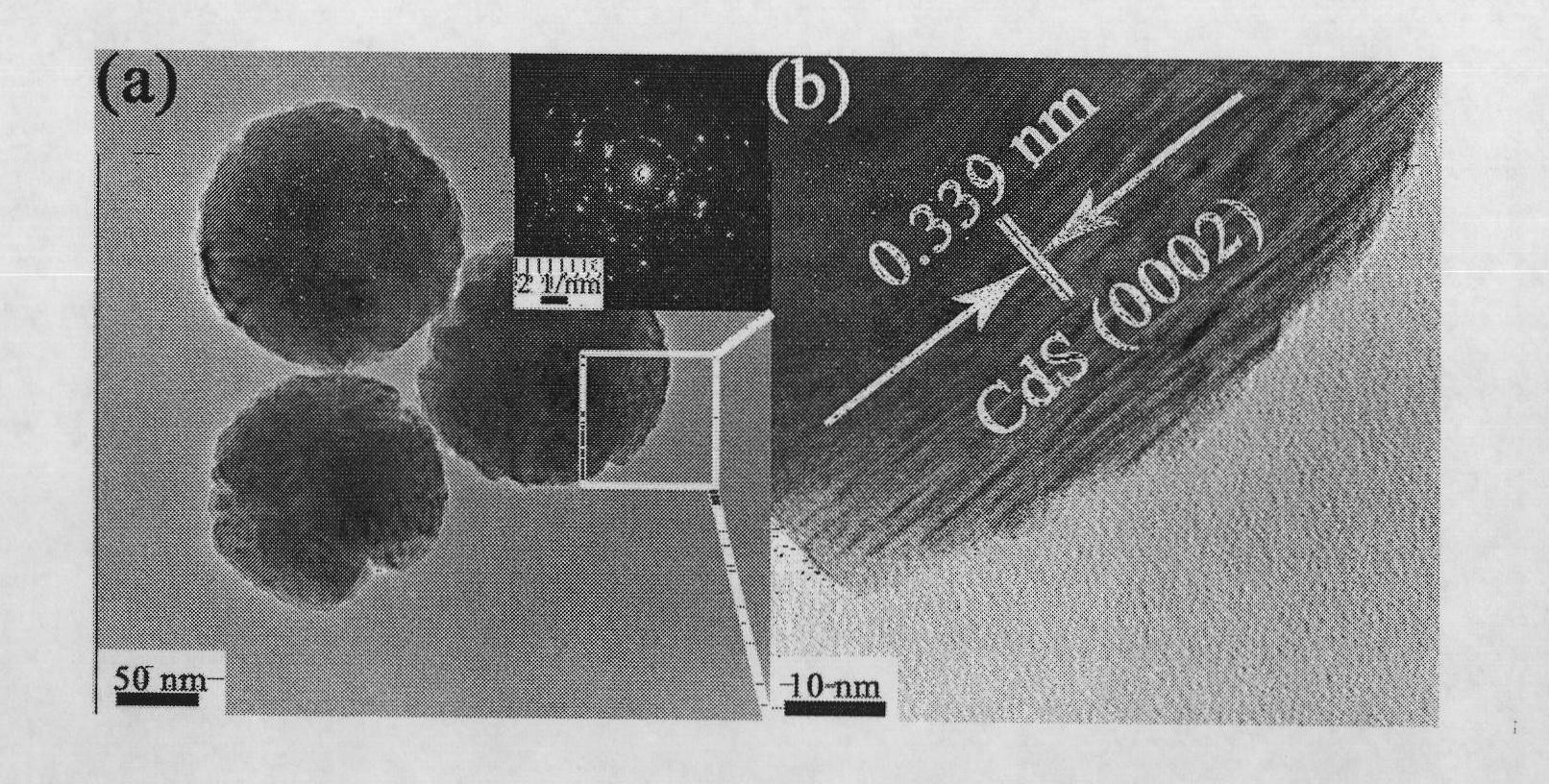
[0034] First weigh 0.0005mol Cd(Ac) 2 2H 2 O (0.1333g) and 0.004mol thiourea (0.3045g), ie thiourea and Cd(Ac) 2 2H 2 O molar ratio is 8. The above weighed Cd (Ac) 2 2H 2 O and thiourea were dissolved together in 50 mL of deionized water, and ultrasonically dissolved for half an hour to form a colorless transparent aqueous solution. The above mixed aqueous solution was transferred to a stainless steel reaction kettle lined with polytetrafluoroethylene (capacity 80 mL), placed in an oven, and reacted at 150° C. for 2 hours. After cooling to room temperature, the bright yellow precipitate was collected by centrifugation (10000rpm, 10min), washed twice with deionized water and absolute ethanol, and finally dried in an oven at 60°C for 6 hours. The prepared flower-like CdS nanocrystalline powder is placed in a desiccator for later use.
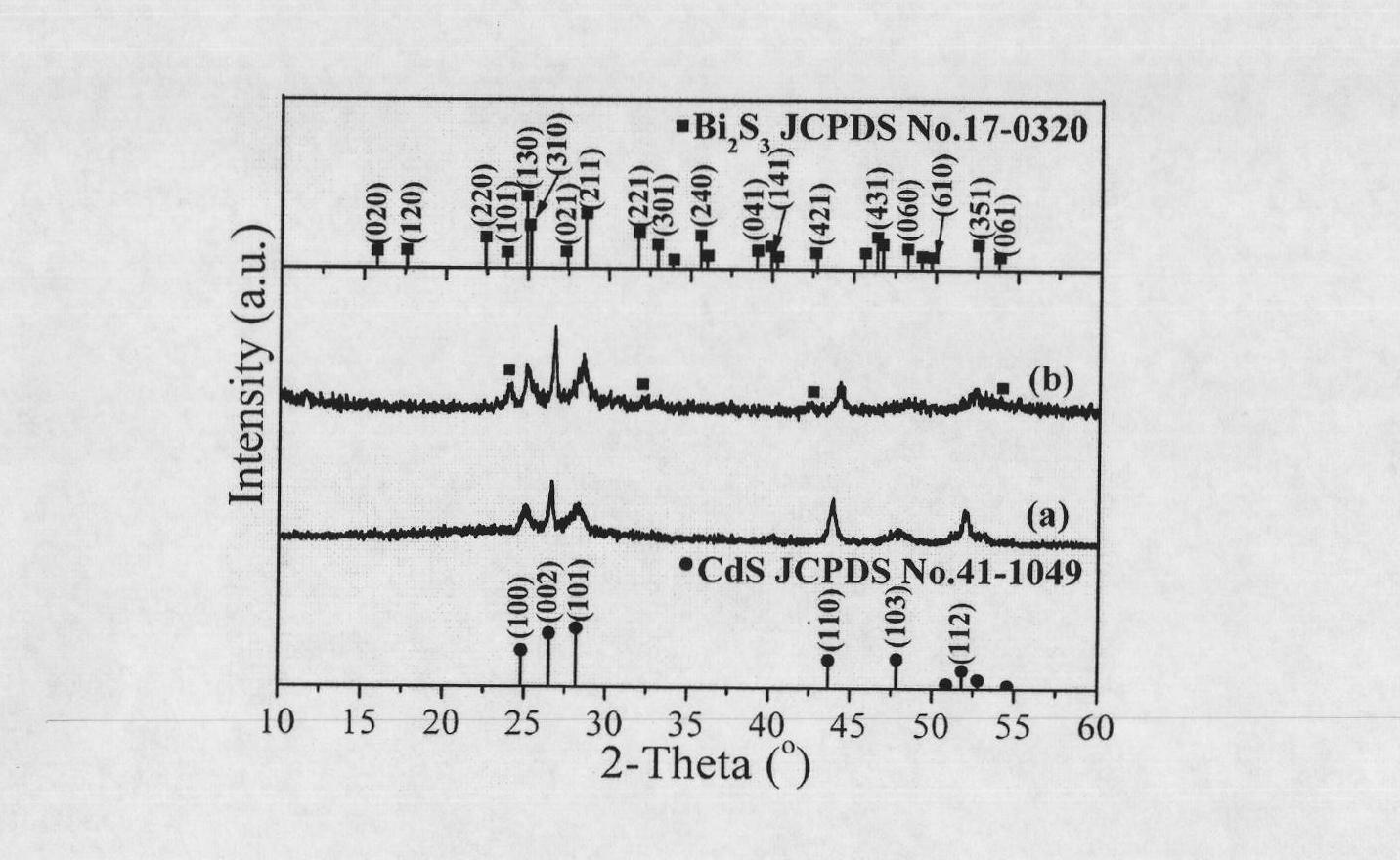
[0035] figure 1 a is the XRD pattern of CdS parent nanocrystals, all the diffraction peaks are consistent with the standard card of wurtz...
Embodiment 2
[0037] Weigh 0.005 g of dried flower-like CdS nanocrystalline powder, then disperse it in 50 g of ethylene glycol, and process it ultrasonically for 1 hour to form a suspension with a milk concentration of 0.0002 M. Mix 20 mL of CTAB ethylene glycol solution with a concentration of 0.005 M and 15 g of the ethylene glycol suspension of the flower-like CdS nanocrystal powder, and stir for 15 min. Add 2mL of Bi(NO 3 ) 3 ·5H 2 O ethylene glycol solution, stirred at room temperature for 15 min, and then placed in an oil bath at 77 ° C for 1.5 hours of heat treatment. After the reaction was over, it was taken out from the oil bath and cooled to room temperature. The precipitate obtained by the reaction was separated from the ethylene glycol solution by centrifugation (10000 rpm, 10 min), and then washed twice with deionized water and absolute ethanol. Finally, it was dried in an oven at 60° C. for 6 hours.
[0038] figure 1 B is the CdS-Bi obtained in Example 2 2 S 3 The XRD...
Embodiment 3
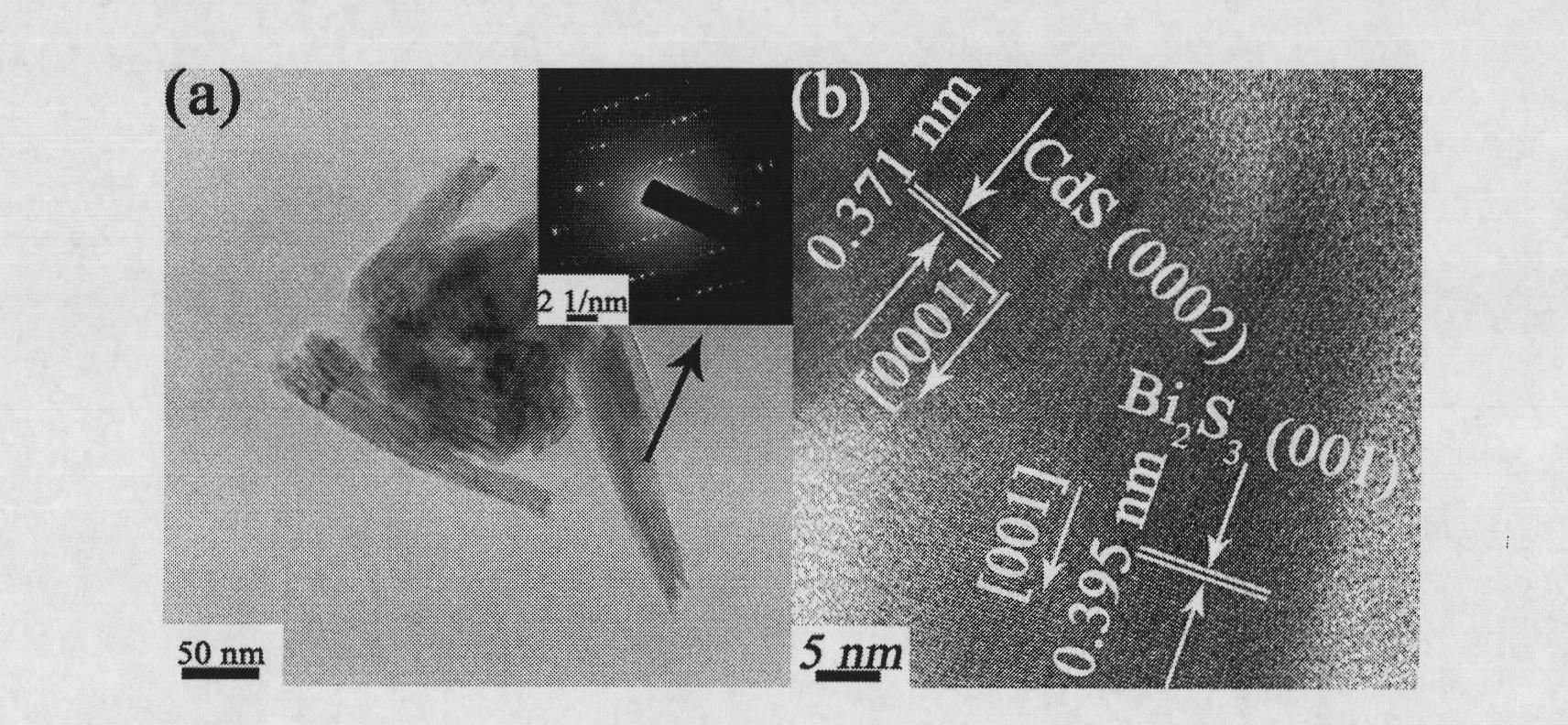
[0040] Weigh 0.005 g of dried flower-like CdS nanocrystalline powder, then disperse it in 50 g of ethylene glycol, and process it ultrasonically for 1 hour to form a suspension with a milk concentration of 0.0002 M. Mix 20 mL of CTAB ethylene glycol solution with a concentration of 0.005 M and 15 g of the ethylene glycol suspension of the flower-like CdS nanocrystal powder, and stir for 15 min. Add 2mL of Bi(NO 3 ) 3 ·5H 2 O ethylene glycol solution, stirred at room temperature for 15 min, and then placed in an oil bath at 90° C. for 1.5 hours. After the reaction was over, it was taken out from the oil bath and cooled to room temperature. The precipitate obtained by the reaction was separated from the ethylene glycol solution by centrifugation (10000 rpm, 10 min), and then washed twice with deionized water and absolute ethanol. Finally, it was dried in an oven at 60° C. for 6 hours. All the other are with embodiment 2. Figure 4 a is the CdS-Bi prepared in this example ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com