Preparation method of borneol diene
A technology for bornadiene and pinene, which is applied in the field of preparing bornadiene, can solve the problems of chlorine reaction metering difficulty, many steps and devices, unstable reaction and the like, and achieves the advantages of industrialized operation, mature technology and reduced production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9
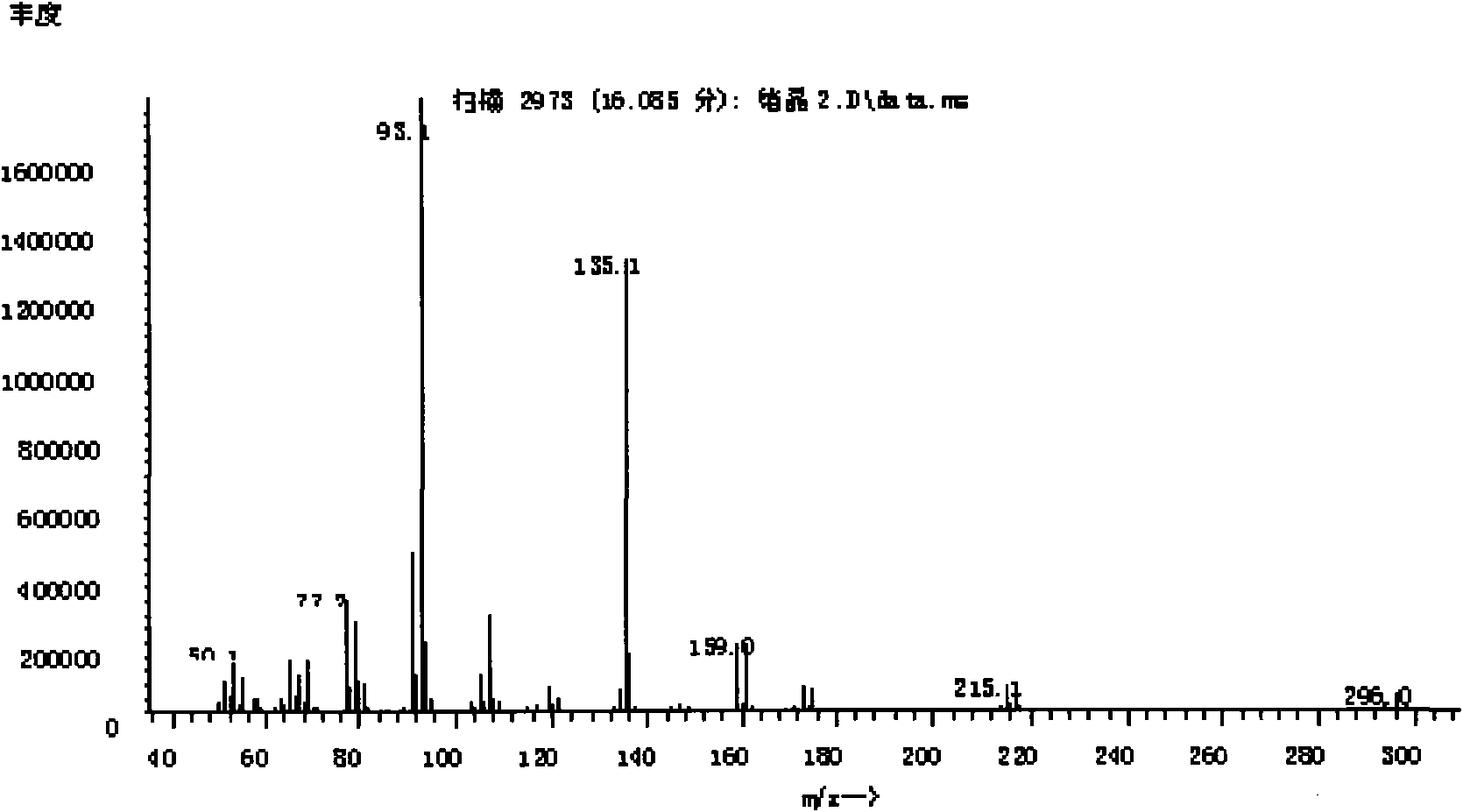
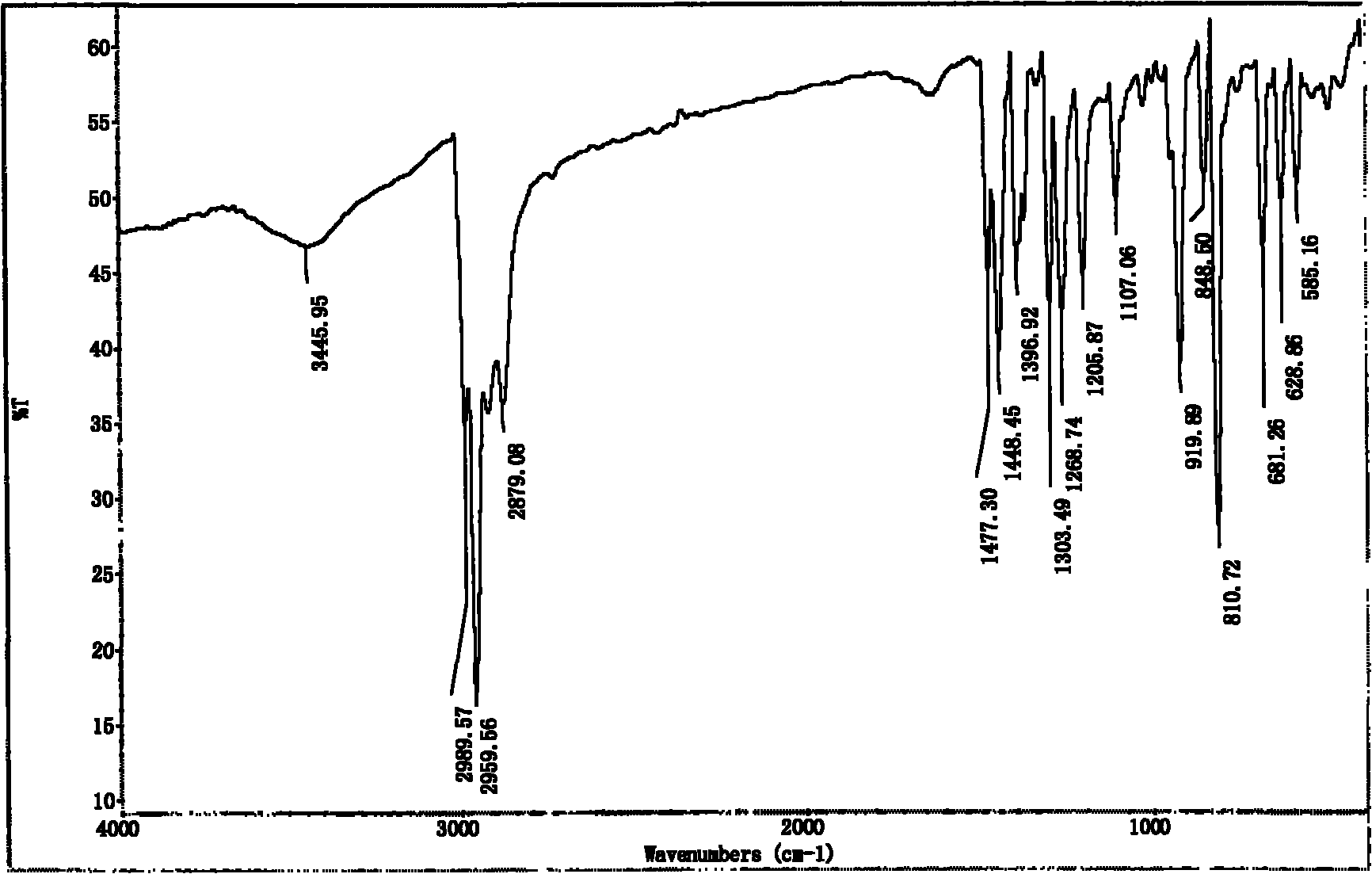
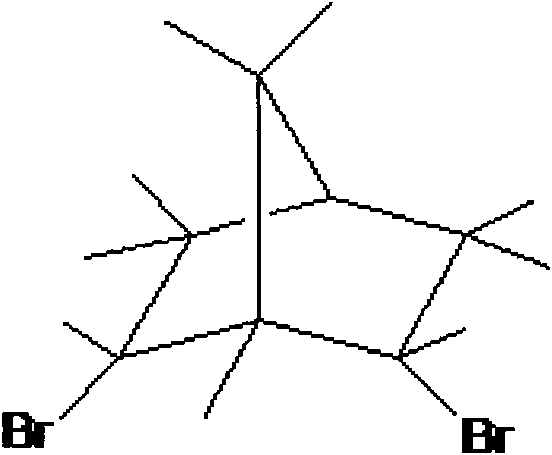
[0024] In step (1), add 10ml α-pinene and 40ml solvent A in a 250mL four-neck flask equipped with a mechanical stirring device, a thermometer, and a reflux condenser, and pre-mix the liquid bromine of 10ml solvent A with a feed ratio C to form a bromine solution , immerse the reaction flask in a low-temperature bath, control the temperature E, and slowly add bromine solution dropwise to the reaction flask through a constant pressure dropping funnel while stirring at a speed of 0.25 drops / min. After the bromine solution has been added dropwise, continue After stirring for a reaction time G, the reaction was terminated; the reaction bottle was taken out to return to room temperature, and NaHCO was added 3 Wash with aqueous solution for at least 2 times, generally 3 to 4 times. At this time, the organic layer solution is light yellow to white and transparent, and then separate the liquids. Take an appropriate amount of anhydrous magnesium sulfate to remove water from the organic l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com