Insecticide composition and preparation method and application thereof
A technology of composition and insecticide, which is applied in the direction of botany equipment and methods, insecticide, application, etc., can solve the problems of inability to make insecticide and poor effect, and achieve good control effect, good drug effect, Small stimulation effect on humans and animals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11R-c
[0042] Example 1.1R-cis(Z)-2,2-dimethyl-3-(2-fluoro-1-propenyl)-cyclopropanecarboxylic acid or acid chloride
[0043] Preparation of 1R-cis(Z)2`2-dimethyl-3-(2-fluoro-1-propenyl)cyclopropanecarboxylic acid
[0044] 0.5 g of p-toluenesulfonic acid (PTSA) was added to dissolved 5.78 g of 1R-dis(Z)-2,2-dimethyl-3-(2-fluoro-1-propenyl)cyclopropanecarboxy-1,1 - Dimethyl ethyl ester and 50 ml of toluene. React until heated to reflux at 120°C. Then cool down to 20 degrees, dilute with isopropyl ether, wash with water, dry, filter and concentrate to obtain 4.40g 1R-cis(Z)-2,2-dimethyl-3-(2-fluoro-1-propenyl) Cyclopropane carboxylic acid.
[0045] Preparation of 1R-cis(Z)2`2-dimethyl-3-(2-fluoro-1-propenyl)-cyclopropanecarboxylic acid chloride
[0046] 1ml DMF and 4.5ml (COCl)2 was added to 0°C to dissolve 4.40g 1R-cis(Z)-2,2-dimethyl-3-(2-fluoro-1-propenyl)cyclopropanecarboxylic acid and 50ml dichloromethane at 0°C Stir at low temperature for 45 minutes, then react at 20°C for 13...
Embodiment 21
[0047] Example 2.1R-trans(E)-2,2-dimethyl-3-(2-fluoro-1-propenyl)-cyclopropanecarboxylic acid or acid chloride
[0048] 1.2 g of APTS was added dissolved in 13.86 g of 1R-trans(E)-2,2-dimethyl-3-(2-fluoro-1-propenyl)cyclopropanecarboxy-1,1-dimethylethyl ester and 140ml of toluene. React until heated to reflux at 120°C. Then cool down to 20°C, dilute with 250ml of isopropyl ether, wash with water, dry, filter and concentrate to obtain 11g of 1R-trans(E)-2,2-dimethyl-3-(2-fluoro-1-propenyl) Cyclopropane carboxylic acid.
[0049] 3ml DMF and 10ml of (COCl) 2 It was added to 0°C to dissolve 10 g of 1R-trans(E)2,2-dimethyl-3-(2-fluoro-1-propenyl)cyclopropanecarboxylic acid and 100 ml of dichloromethane. Stir at 0°C for 45 minutes, then react at 20°C for 135 minutes, concentrate and recover the solvent dichloromethane at 40°C, dissolve the distillate in toluene, concentrate and dry, then add 55ml to form an acid chloride toluene solution, so that 1M / L acid chloride Toluene solu...
Embodiment 3
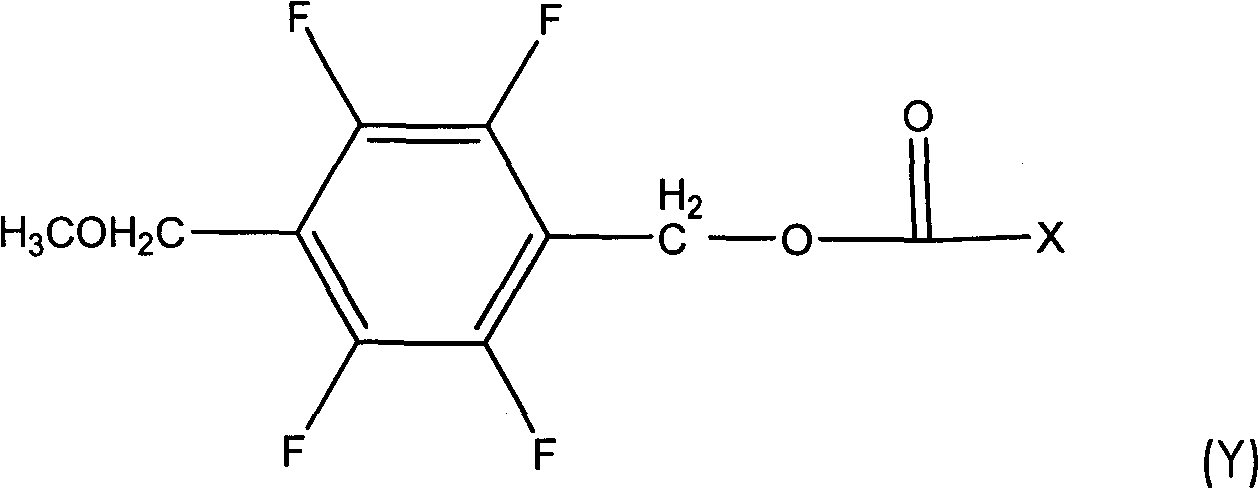
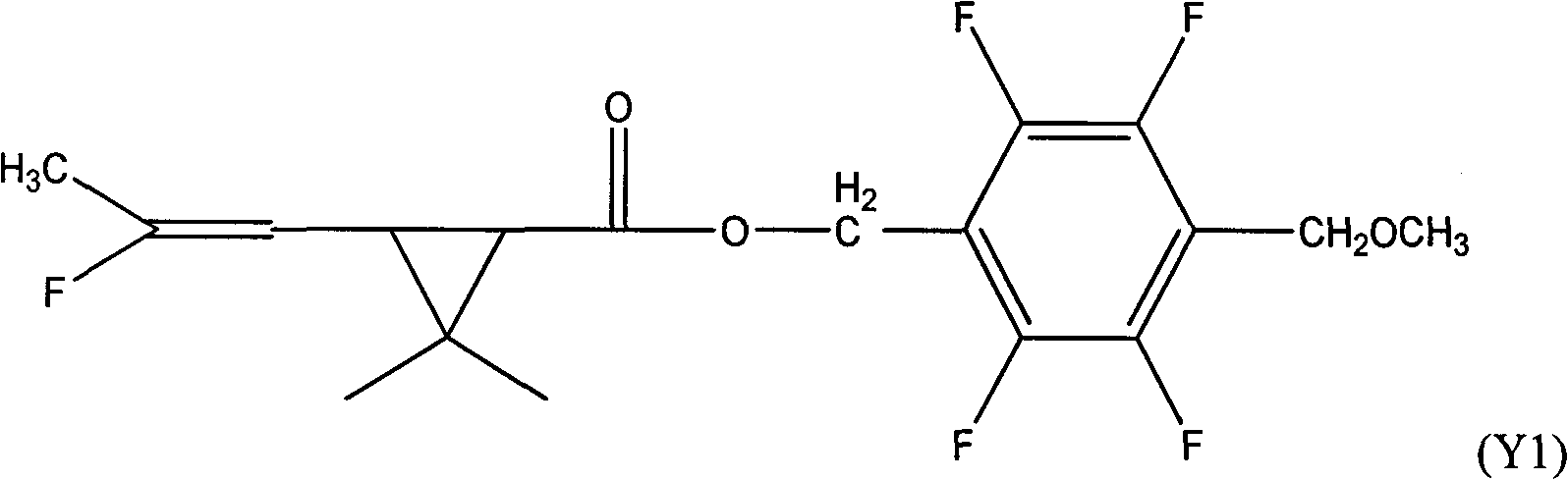
[0052] In a 500ml four-necked reaction flask, put 52.5g of 2,3,5,6-tetrafluoro-4-methoxybenzyl alcohol, 25.0g of pyridine, dissolve in 200ml of toluene, stir well, drop at 5~10℃ Add 48 g of 3-(2-fluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid chloride, and after the dropwise addition, raise the temperature to 25° C. for 2 hours to react. Wash twice with 150ml 10% hydrochloric acid, then wash with 150ml 10% NaHCO 3 Wash twice, separate the toluene layer and heat at 0.08Mpa to remove the solvent toluene to obtain 2,3,5,6-tetrafluoro-4-methoxybenzyl-3-(2-fluoro-1-propenyl) -2,2-dimethylcyclopropane carboxylate, the weight is 85.7g, the content is 91.5.0%, and the yield is 86.2%.
[0053]
[0054] The IR spectrum shows υ-c=o 1750cm -1 The strong absorption peak at and υ-c-o-c-1180cm -1 and 1160cm -1 The two strong peaks of the product indicate the presence of ester groups; υ-c=o at 1720cm -1 The strong and broad peaks on the left and right indicate the presence ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com