Method for selectively replicating replication-defective adenovirus and application
A replication-defective, adenovirus technology, applied in the field of biomedicine, can solve the problems of danger, ineffective gene therapy, low activity, etc., and achieves the effect of overcoming safety problems and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
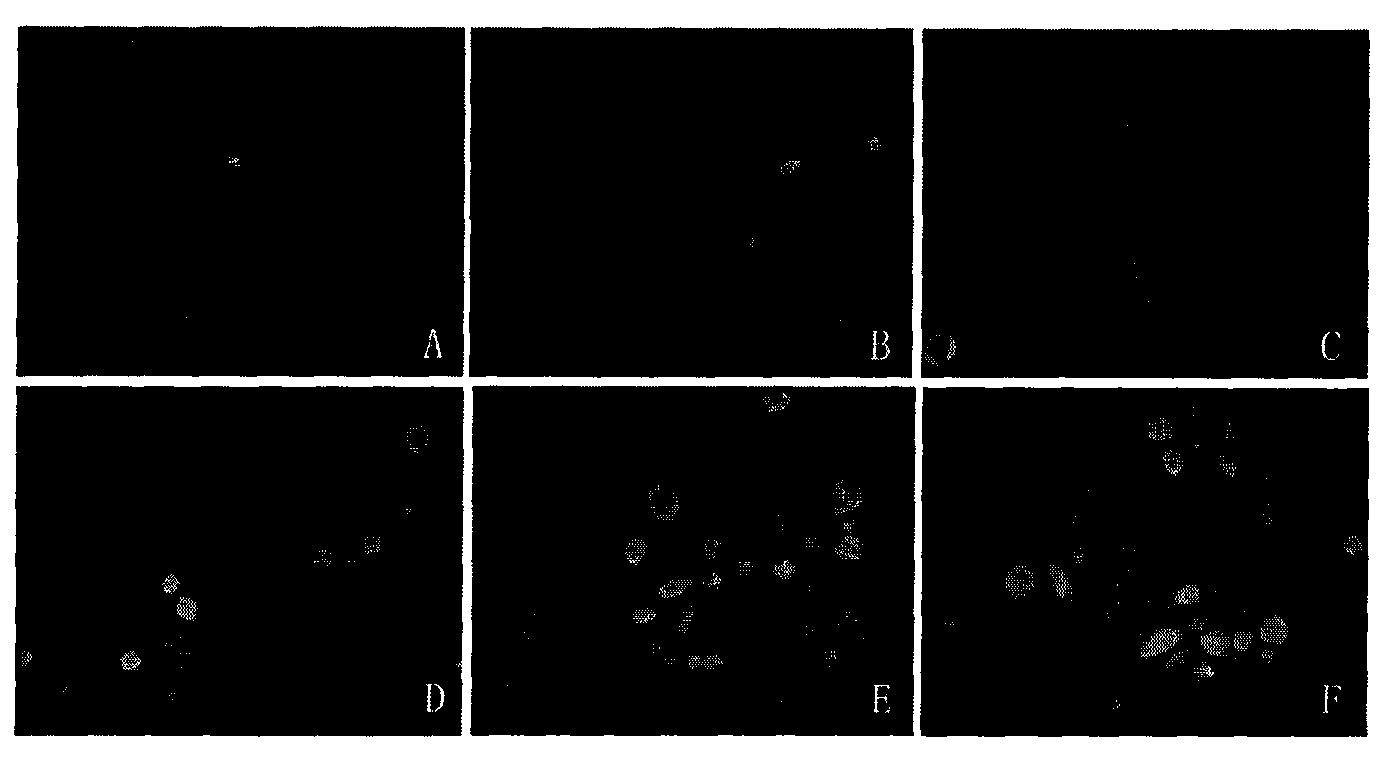
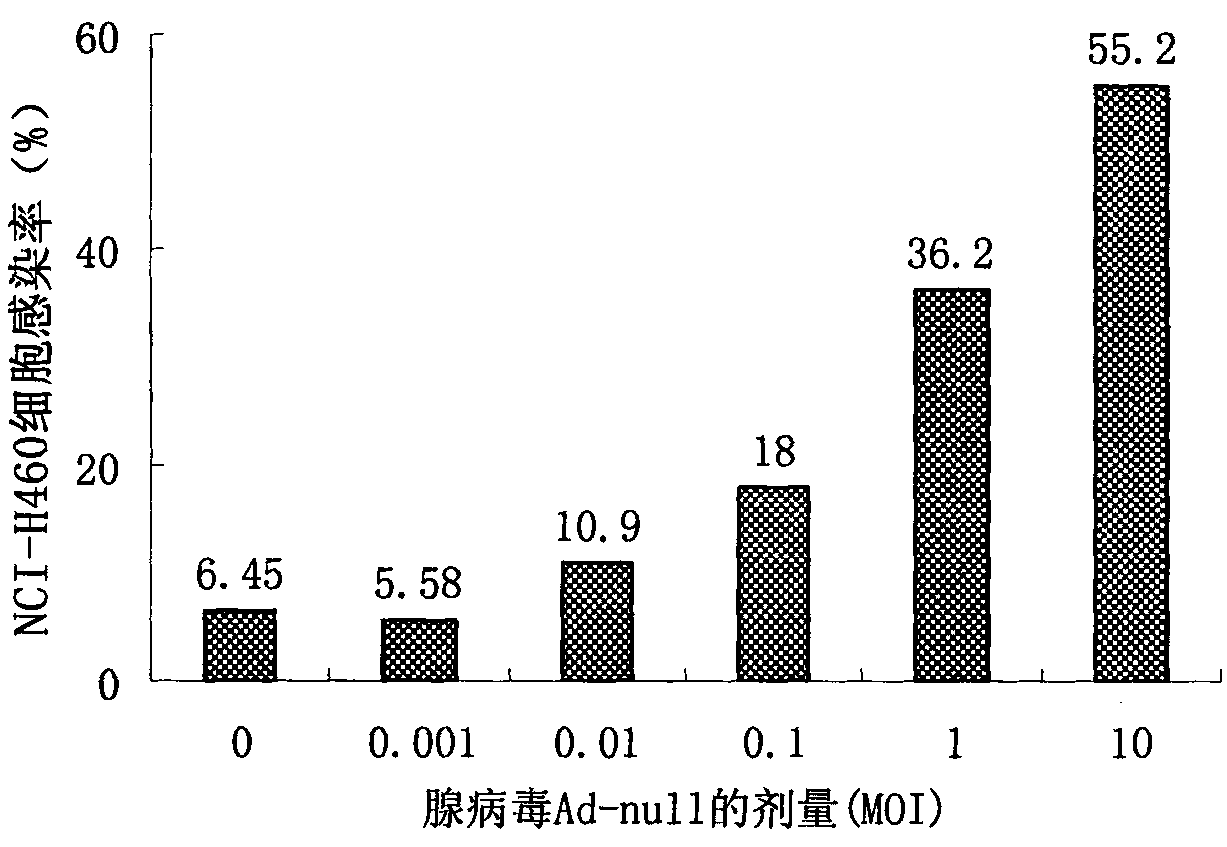
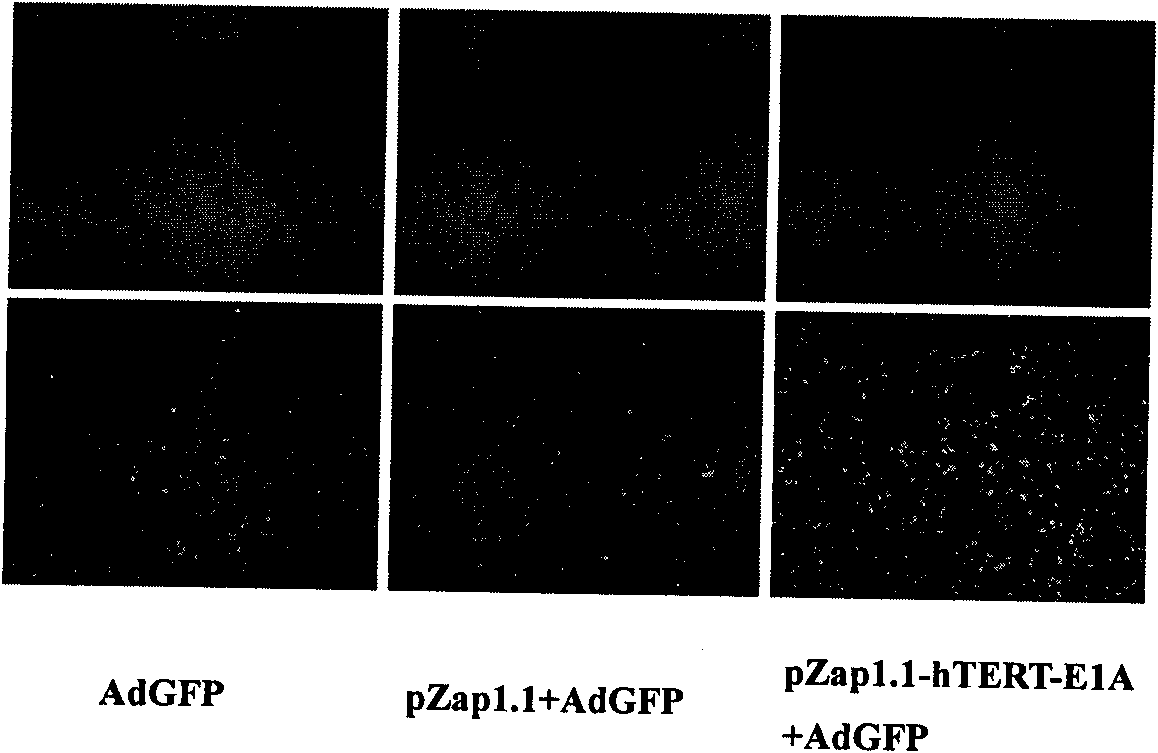
Image
Examples
Embodiment 1
[0061] Isolation of tumor-specific promoters by PCR reaction and cloning
[0062] A. The regulatory sequence at the 5' end of telomerase
[0063]During natural DNA replication, the ends of chromosomes shorten with each cell division. The integrity of telomeres at the end of chromosomes is crucial to the stability of chromosomes. The integrity of telomeres is maintained by telomerase. Telomerase is inactive in more than 99% of normal cells and is reactivated in more than 90% of tumor cells. Experiments have shown that the activity of telomerase is controlled by its promoter. The promoter of telomerase (TERT) is active in most tumors but not in normal cells. Thus, the promoter of TERT becomes a tumor-selectively activated promoter. In order to isolate the telomerase promoter, the present invention uses PCR technology to amplify the promoter (sequence one) using human cell DNA as a template, and insert it into the plasmid pEGFP-1 (purchased from Clontech Company) to drive th...
Embodiment 2
[0079] A eukaryotic expression vector carrying a tumor-specific promoter regulating the E1A gene, an essential element for adenovirus replication, was constructed.
[0080] The tumor-specific promoter (TSP) isolated in Example 1 was embedded into a eukaryotic expression vector by genetic engineering, and used to control the expression of the early gene E1A necessary for adenovirus replication. The characteristic of the plasmid is that the adenovirus replication essential element E1A gene carried by it is regulated by a tumor tissue-specific promoter. The structure of the eukaryotic expression vector used in the present invention is schematically shown as: Plasmid-TSP-E1A, wherein Plasmid is any eukaryotic expression plasmid (such as: pcDNA3.1); TSP is the tumor-specific isolated in Example 1 promoter and other tumor-specific promoters not mentioned.
Embodiment 3
[0082] To construct an adeno-associated virus (rAAV) vector carrying a tumor-specific promoter regulating the essential element E1A gene for adenovirus replication.
[0083] The tumor-specific promoter (TSP) isolated in Example 1 was embedded into the shuttle plasmid of the adeno-associated virus by genetic engineering, and used to control the expression of the early gene E1A necessary for the replication of the adenovirus. The characteristic of the plasmid and virus is that the adenovirus replication essential element E1A gene carried by them is regulated by a tumor tissue-specific promoter. The shuttle plasmid structure of the adeno-associated virus used in the present invention is schematically shown as pSNAV-TSP-E1A, wherein, pSNAV is a general-purpose shuttle plasmid for packaging different serotypes of AAV, and can be used for packaging any serotype of adeno-associated virus; TSP is Example 1 Tumor-specific promoters isolated in and other tumor-specific promoters not men...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com