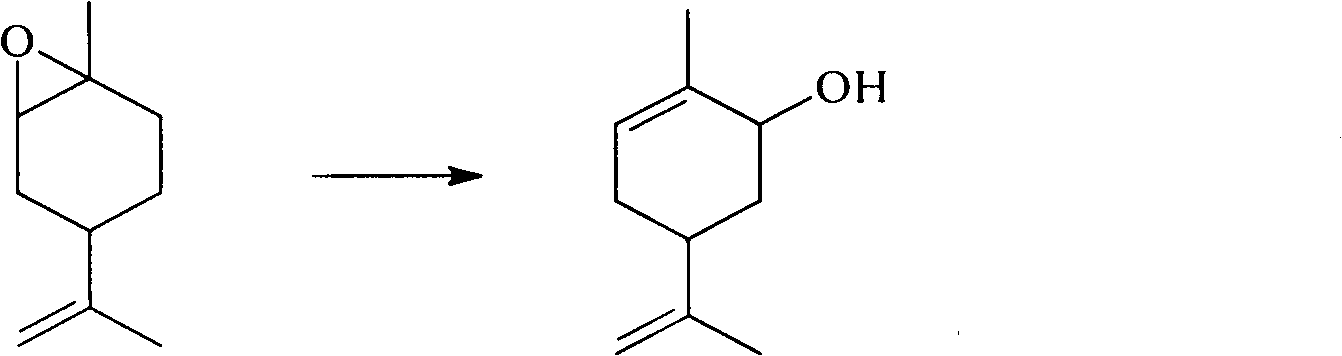
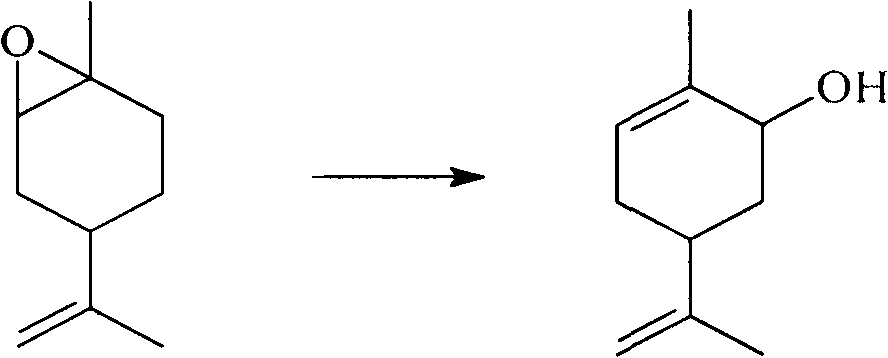
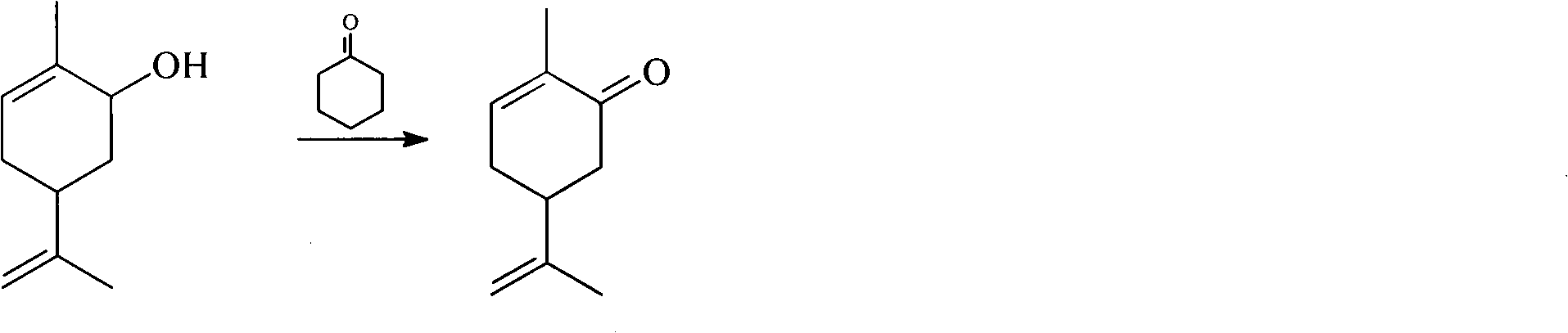Method for synthesizing carvone
A synthesis method and technology of carvone, applied in the field of synthesis of spices and spices, can solve the problems of complex process, high concentration, low total yield, etc., and achieve the effect of economical and environmental protection of the synthesis process, simple and easy-to-obtain raw materials, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In a 100 ml three-necked flask, add 50.0 grams of epoxy limonene (content 97%), 0.4 grams of 2-aminophenol (n / n: 86:1), stir with a magnetic stirrer, and heat to an internal temperature of 180 to 190 °C, after 4-6 hours, the rearrangement reaction was completed, and 46.7 g of crude carveol was obtained by distillation under reduced pressure, and then the finished product of carveol was obtained after rectification. The conversion rate of epoxylimonene was 85%, and the selectivity of carveol was 89%.
Embodiment 2
[0024] In a 100 ml three-necked flask, add 50.0 grams of limonene epoxy (content 97%), 2.1 grams of zinc octoate (n / n: 86:1), stir with a magnetic stirrer, and heat to an internal temperature of 180 to 190 ° C. After 4 to 6 hours, the rearrangement reaction was completed, and 42.3 g of crude carveol was obtained by distillation under reduced pressure, and the finished product of carveol was obtained after rectification. The conversion rate of epoxylimonene was 85.8%, and the selectivity of carveol was 81.2%.
Embodiment 3
[0026] In a 100 ml three-necked flask, add 50.0 g of limonene epoxy (content 97%) and 1.53 g of zinc octoate (n / n: 63:1), stir with a magnetic stirrer, and heat to an internal temperature of 205-210°C. After 4 to 6 hours, the rearrangement reaction was completed, and 43.7 g of crude carveol was obtained by distillation under reduced pressure, and the finished product of carveol was obtained after rectification. The conversion rate of epoxylimonene was 87.1%, and the selectivity of carveol was 83.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com