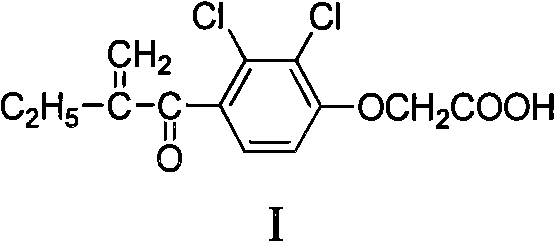
Alpha, beta-unsaturated ketone compound containing 1,2,4-oxadiazoles heterocycle
A compound, the technology of oxadiazole, which is applied in the field of anti-tumor activity containing 1, can solve problems such as limiting clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
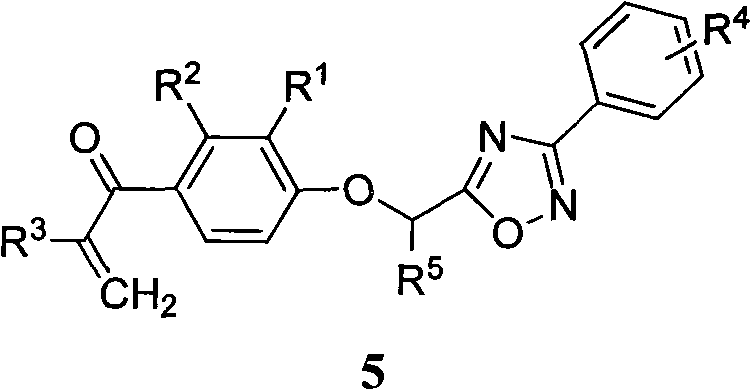
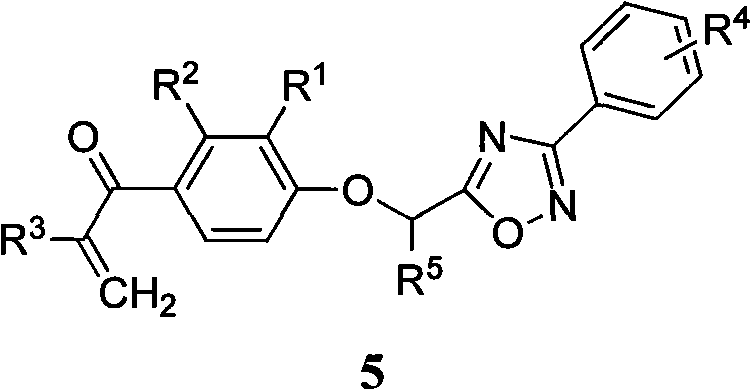
[0034] The preparation method of the general formula 5 containing 1,2,4-oxadiazole α,β-unsaturated ketone compound of the present invention, the steps are as follows:
[0035] 1) Preparation of substituted amidoxime (2)
[0036] Add hydroxylamine hydrochloride to anhydrous methanol, add 0.8mL of anhydrous methanol to every millimole of hydroxylamine hydrochloride, then add anhydrous potassium carbonate, react at room temperature until no bubbles are formed, then add substituted benzonitrile (1). The molar ratio of hydroxylamine hydrochloride, anhydrous potassium carbonate and substituted benzonitrile is 5:5:1. Reflux reaction for 4-7h, filter while hot, wash the filter cake with anhydrous methanol, combine the washing liquid and filtrate, evaporate the solvent under reduced pressure, and separate the substituted amidoxime (2) by column chromatography, the elution system is petroleum ether / acetone 3:1 volume ratio.
[0037] 2) Preparation of 2-[2,3-disubstituted-4-(2-methylen...
Embodiment 1
[0051] 1) Preparation of substituted benzamide oxime compound (2)
[0052] Put 3.48g (50mmol) of hydroxylamine hydrochloride and 6.90g (50mmol) of anhydrous potassium carbonate in a 100mL round bottom flask, add 40mL of anhydrous methanol, and react at room temperature until no bubbles are generated, then add 1.71 g of p-substituted benzonitrile (5c) g (10 mmol). Slowly raise the temperature to 65°C, react for 4-7h, filter while hot, wash the filter cake with anhydrous methanol, combine the washing liquid and filtrate, remove the solvent by rotary evaporation, dry, and separate by column chromatography to obtain the intermediate substituted benzamide oxime, The elution system is petroleum ether / acetone 3:1 volume ratio.
[0053] Substituted benzonitrile selects 4-trifluoromethylbenzonitrile, 2-methylbenzonitrile or 4-nitrobenzonitrile respectively, obtains the following substituted benzamide oxime compound (2) respectively:
[0054] 2a: 4-trifluoromethylbenzamide oxime, whit...
Embodiment 2
[0080] Example 2. Compound GST P1-1 Inhibitory Activity Test and Determination of Growth Inhibition of HL-60 Cells
[0081] GST P1-1 inhibitory activity assay method: refer to CN1706789 "α, β-unsaturated ketone compound and its preparation method and its inhibition of GSTπ activity" 200510043573.3, Example 5 on page 11 of the specification.
[0082] HL-60 cell growth inhibitory activity assay method: refer to CN101108832 "Five-membered Heterocyclic Compounds, Preparation Method and Application", 200710015199.5, page 3 of the description. Human Acute Promyelocytic Leukemia Cell Line (HL-60)
[0083] Table 1. Determination data of compounds inhibiting GST P1-1 activity and inhibiting HL-60 cell growth activity
[0084]
[0085]
[0086] Experimental results show that most of the 1,2,4-oxadiazole α,β-unsaturated ketone compounds of the present invention have a half-inhibitory concentration of HL-60 cells less than or equal to 2.0 μM, and the activity is further improved. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com