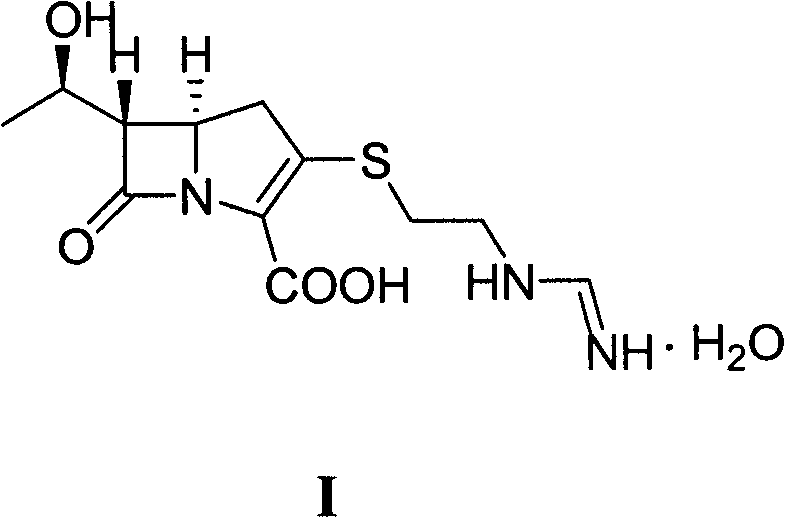
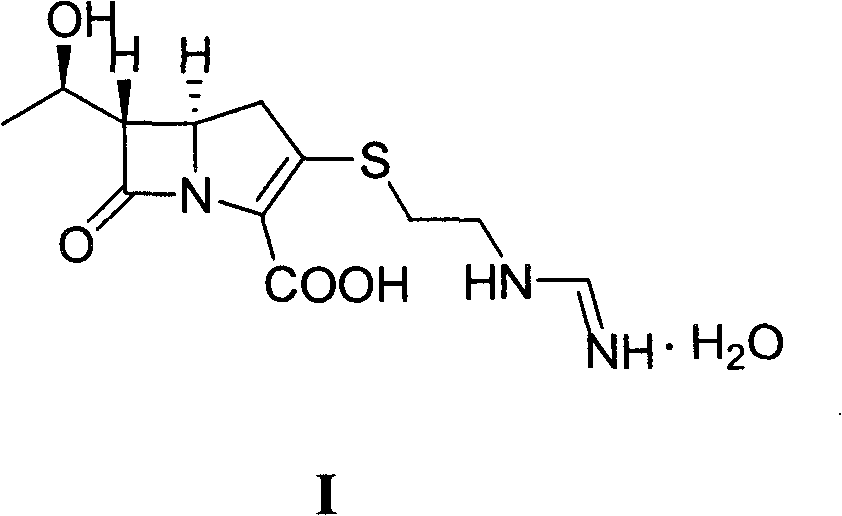
Method for preparing imipenem monohydrate crystal
A monohydrate, imipenem technology, applied in organic chemistry and other directions, can solve problems such as solubility that is not involved, and achieve the effects of easy control, high purity, rapidity, and uniform crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Under nitrogen atmosphere, distilled water (200ml) was heated to 45°C-50°C. The crude imipenem (4.0 g, test = 92%) was added to distilled water, stirred at 45°C-50°C for 2 minutes, 1.2 g of activated carbon was added, and then rapidly cooled to 5°C-10°C. Filter the activated charcoal. Add acetone (100ml) to the filtrate, cool down to -5°C--20°C, and freeze the system. Vigorous stirring was maintained at this temperature for 30 minutes. The temperature of the system was then raised to 5°C-10°C, at which temperature acetone (300ml) was added. Then lower the temperature to 0°C-5°C and stir for 3-4 hours. The crystalline solid was filtered, washed with acetone, and dried under reduced pressure at 40°C for 3-4 hours to obtain white crystal imipenem monohydrate (2.80g, test external standard content 98.5%, dissolution time 10 Second).
[0036] The physical characterization data of the obtained imipenem monohydrate:
[0037] UV max H2O nm: 298
[0038] NMR (δ, D 2 O)...
Embodiment 2
[0041] Under nitrogen atmosphere, distilled water (250ml) was heated to 30°C-35°C. The crude imipenem (4.0 g, test = 92%) was added to distilled water, stirred at 30°C-35°C for 2 minutes, 1.2 g of activated carbon was added, and then rapidly cooled to 5°C-10°C. Filter the activated charcoal. Acetone (125ml) was added to the filtrate, the temperature was lowered to -5°C--20°C, and the system was frozen. Vigorous stirring was maintained at this temperature for 30 minutes. The temperature of the system was then raised to 5°C-10°C, at which temperature acetone (375ml) was added. Then lower the temperature to 0°C-5°C and stir for 3-4 hours. The crystalline solid was filtered, washed with acetone, and dried under reduced pressure at 40°C for 3-4 hours to obtain white crystal imipenem monohydrate (2.40g, external standard content 98.7%, dissolution time 15 Second).
Embodiment 3
[0043] The method of Example 1 was repeated using crude imipenem (4.0 g, assay = 80%) to obtain imipenem monohydrate (2.60 g, assay 98.0%, dissolution time 50 seconds) as white crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com