Condensed aminodihydrothiazine derivative
An amino, thiazide technology, applied in Alzheimer's type dementia, fused aminodihydrothiazine derivatives and their drug applications, the field of Down syndrome, can solve the problem that basic drugs have not yet been developed and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
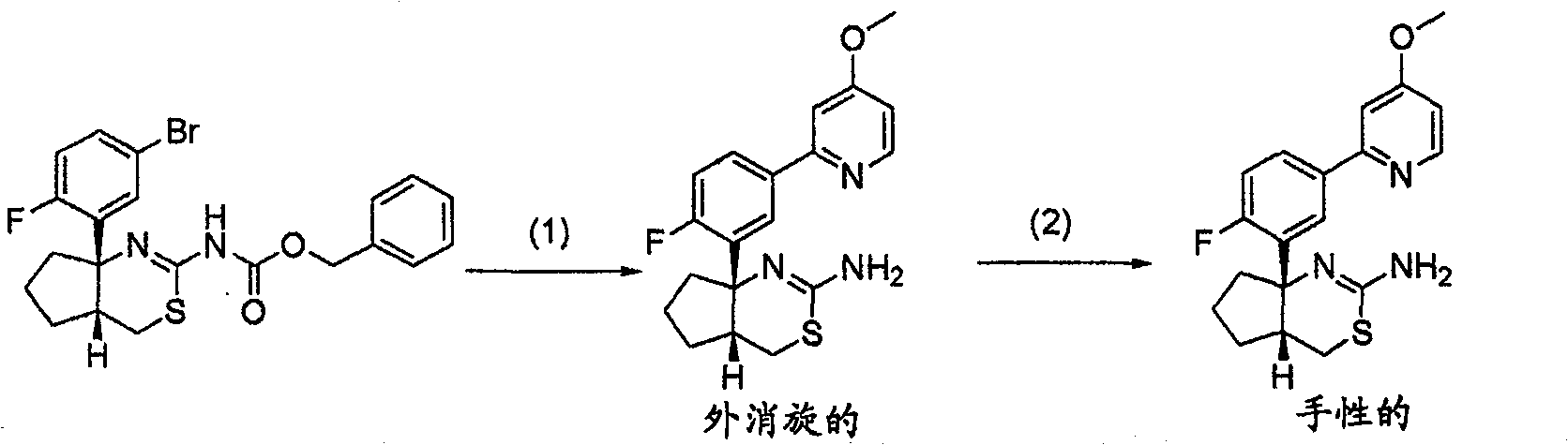
[0524] ( ± )-[(4aR * , 8aS * )-8a-(5-amino-2-fluorophenyl)-4a,5,6,7,8,8a-hexahydro-4H-benzo[d][1,3]thiazin-2-yl] Synthesis of tert-butyl carbamate
[0525] [Formula 20]
[0526]
[0527] (1) Synthesis of Ethyl 2-Trifluoromethanesulfonyloxycyclohex-1-enecarboxylate
[0528] Diisopropylethylamine (38.0 mL) was added to a solution of ethyl 2-oxocyclohexanecarboxylate (8.00 g) in dichloromethane (100 mL) at -78°C under nitrogen atmosphere. After stirring at the same temperature for 10 minutes, trifluoromethanesulfonic anhydride (8.80 mL) was added. The mixture was stirred overnight while gradually warming to room temperature. The mixture was washed with water and twice with 5% aqueous citric acid (150 mL). The organic layer was dried with anhydrous magnesium sulfate. The drying agent was removed by filtration, and the filtrate was concentrated under reduced pressure to give the title compound as a crude product (15.5 g). The crude product was used in the next r...
preparation example 2
[0552] [(4aR * , 8aS * )-8a-(5-amino-2-fluorophenyl)-4a,5,6,7,8,8a-hexahydro-4H-benzo[d][1,3]thiazin-2-yl] Synthesis of tert-butyl carbamate
[0553] [Formula 21]
[0554]
[0555] (1)( +)-[(4aR * , 8aS * )-8a-(2-Fluoro-5-nitrophenyl)-4a,5,6,7,8,8a-hexahydro-4H-benzo[d][1,3]thiazin-2-yl ] Synthesis of tert-butyl carbamate
[0556] CHIRALPAK prepared with Daicel Chemical Industries, Ltd. TM OJ-H optical resolution of the compound (80.0 mg) obtained in Preparation Example 1-(7) (2 cm×25 cm, mobile phase: hexane:ethanol=8:2, flow rate: 20 mL / min). Fractions with retention times of 9.38-18.3 minutes were collected to give the title compound. The same operation was repeated to obtain the title compound (433 mg; >99% ee) from the racemate (1.00 g).
[0557] (2) [(4aR * , 8aS * )-8a-(5-amino-2-fluorophenyl)-4a,5,6,7,8,8a-hexahydro-4H-benzo[d][1,3]thiazin-2-yl] Synthesis of tert-butyl carbamate
[0558] A solution of sodium hydrosulfite (923 mg) ...
preparation example 3
[0561] (-)-[(4aR * , 7aS * )-7a-(5-amino-2-fluorophenyl)-4,4a,5,6,7,7a-hexahydrocyclopentadieno[d][1,3]thiazin-2-yl] Synthesis of tert-butyl carbamate
[0562] [Formula 22]
[0563]
[0564] (1) Synthesis of Ethyl 2-Trifluoromethanesulfonyloxycyclopent-1-enecarboxylate
[0565] N,N-Diisopropylethylamine (27.2 mL) was added to a solution of ethyl 2-oxo-cyclopentanecarboxylate (5.00 g) in dichloromethane (100 mL) at -78°C for 10 minutes. Trifluoromethanesulfonic anhydride (5.92 mL) was added dropwise to the reaction solution at the same temperature. The reaction solution was stirred overnight while gradually warming to room temperature. Water was added to the reaction mixture, which was then washed twice with 5% aqueous citric acid (150 mL). The organic layer was dried with anhydrous magnesium sulfate. The drying agent was removed by filtration and toluene (200 mL) was added to the filtrate. Dichloromethane was evaporated under reduced pressure at room tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com