Production method of 2,3,3,3-tetrafluoropropene
A technology of tetrafluoropropene and its production method, which is applied in the production field of 2,3,3,3-tetrafluoropropene, can solve the problems of easy coking and deactivation of the catalyst, short service life, short continuous production cycle, etc., and achieve the extension of continuous production cycle, the effect of inhibiting coking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
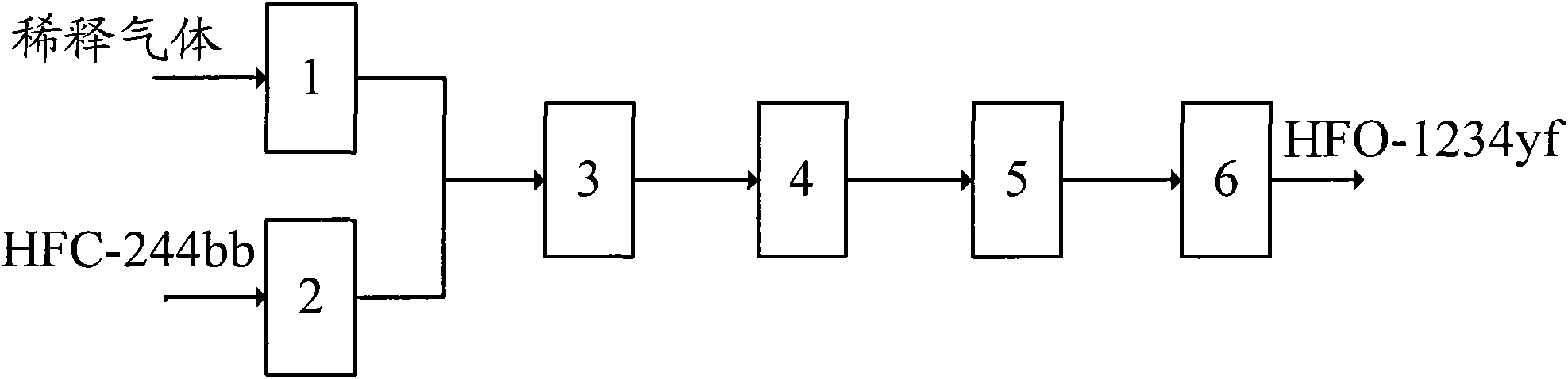
[0037] In this embodiment, the pyrolysis reactor 4 adopts an external heating reactor with a pipe diameter of ∮19×2mm, and the diluent gas is nitrogen. Its technological process is:
[0038] A. Preheat the nitrogen to 600°C through the dilution gas preheating device 1; at the same time, preheat the HCFC-244bb to 200°C through the HCFC-244bb preheating device 2;
[0039] B, after preheating, nitrogen and HCFC-244bb enter material mixing device 3, and mix according to the mol ratio of nitrogen and HCFC-244bb as 20: 1;
[0040] C, the mixture of nitrogen and HCFC-244bb obtained in step B enters the tubular thermal cracking reactor 4, and performs thermal cracking reaction at a reaction temperature of 450 ° C and a residence time of 60 seconds;
[0041] D. The cracking reaction product is cooled to 50°C through the quenching treatment device;
[0042] E. Post-processing
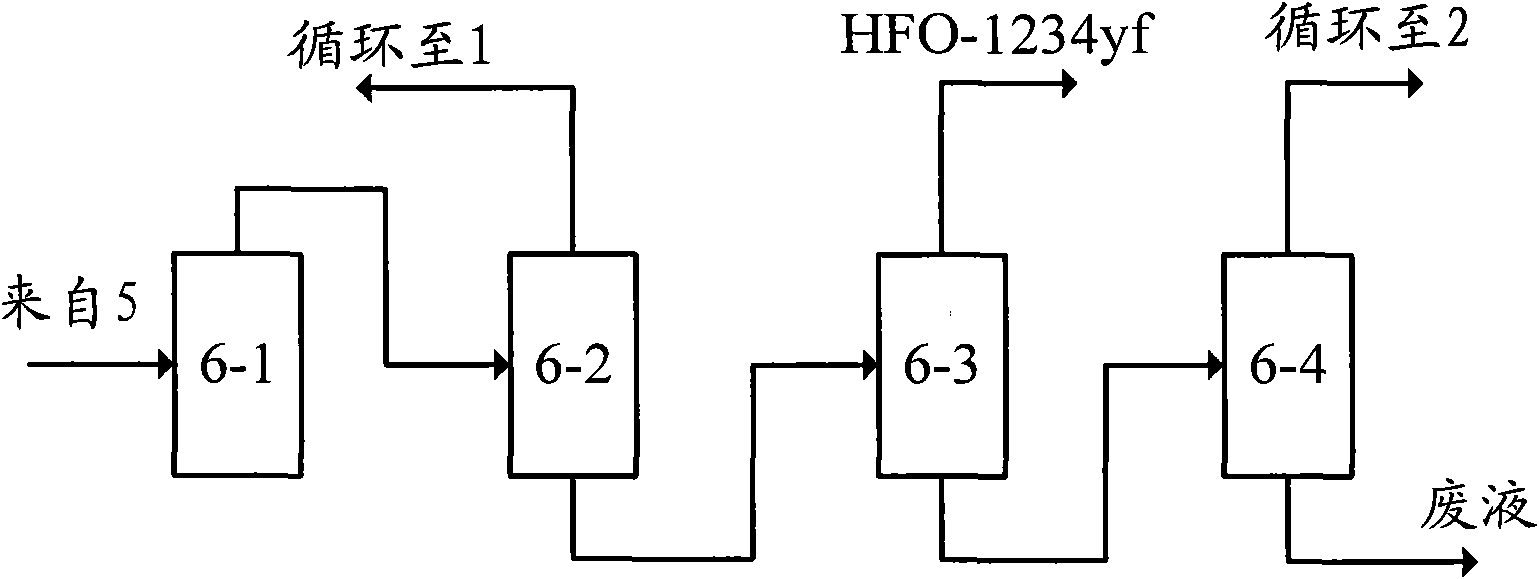
[0043] E-a, acid removal: the product obtained in step D enters the water alkali washing acid removal devic...
Embodiment 2
[0049] In this embodiment, the pyrolysis reactor 4 adopts an external heating reactor with a pipe diameter of ∮19×2 mm, and the diluent gas is water vapor. Its technological process is:
[0050] A. The water vapor is preheated to 1100°C through the dilution gas preheating device 1; at the same time, the HCFC-244bb is preheated to 300°C through the HCFC-244bb preheating device 2;
[0051] B, steam and HCFC-244bb after preheating enter material mixing device 3, mix according to the mol ratio of steam and HCFC-244bb be 5: 1;
[0052]C. The mixture of water vapor and HCFC-244bb obtained in step B enters the tubular thermal cracking reactor 4, and performs thermal cracking reaction at a reaction temperature of 900°C and a residence time of 0.01 seconds;
[0053] D. The pyrolysis reaction product is cooled to 150°C by the quenching device;
[0054] E. Post-processing
[0055] E-a, dehydration, acid removal: the product obtained in step D is dehydrated by low-pressure condensation...
Embodiment 3
[0060] In this embodiment, the pyrolysis reactor 4 adopts an external heating reactor with a pipe diameter of ∮19×2 mm, and the diluent gas is HF. Its technological process is:
[0061] A. Preheat HF to 900°C through dilution gas preheating device 1; at the same time, preheat HCFC-244bb to 300°C through HCFC-244bb preheating device 2;
[0062] B, after preheating, HF and HCFC-244bb enter material mixing device 3, and mix according to the molar ratio of HF and HCFC-244bb is 1: 1;
[0063] C. The mixture of HF and HCFC-244bb obtained in step B enters the tubular thermal cracking reactor 4, and performs thermal cracking reaction at a reaction temperature of 600°C and a residence time of 0.5 seconds;
[0064] D. The cracking reaction product is cooled to 100°C through the quenching treatment device;
[0065] E. Post-processing
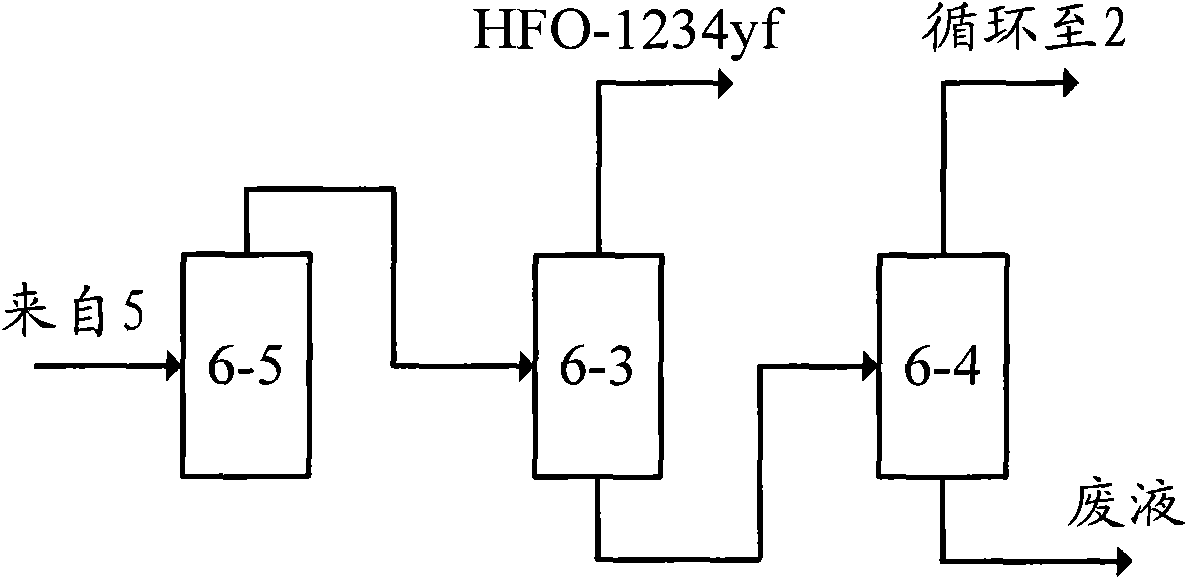
[0066] E-a. Separation and acid removal: first, the product obtained in step D is separated through the separation tower 6-6, and the overhead fracti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com