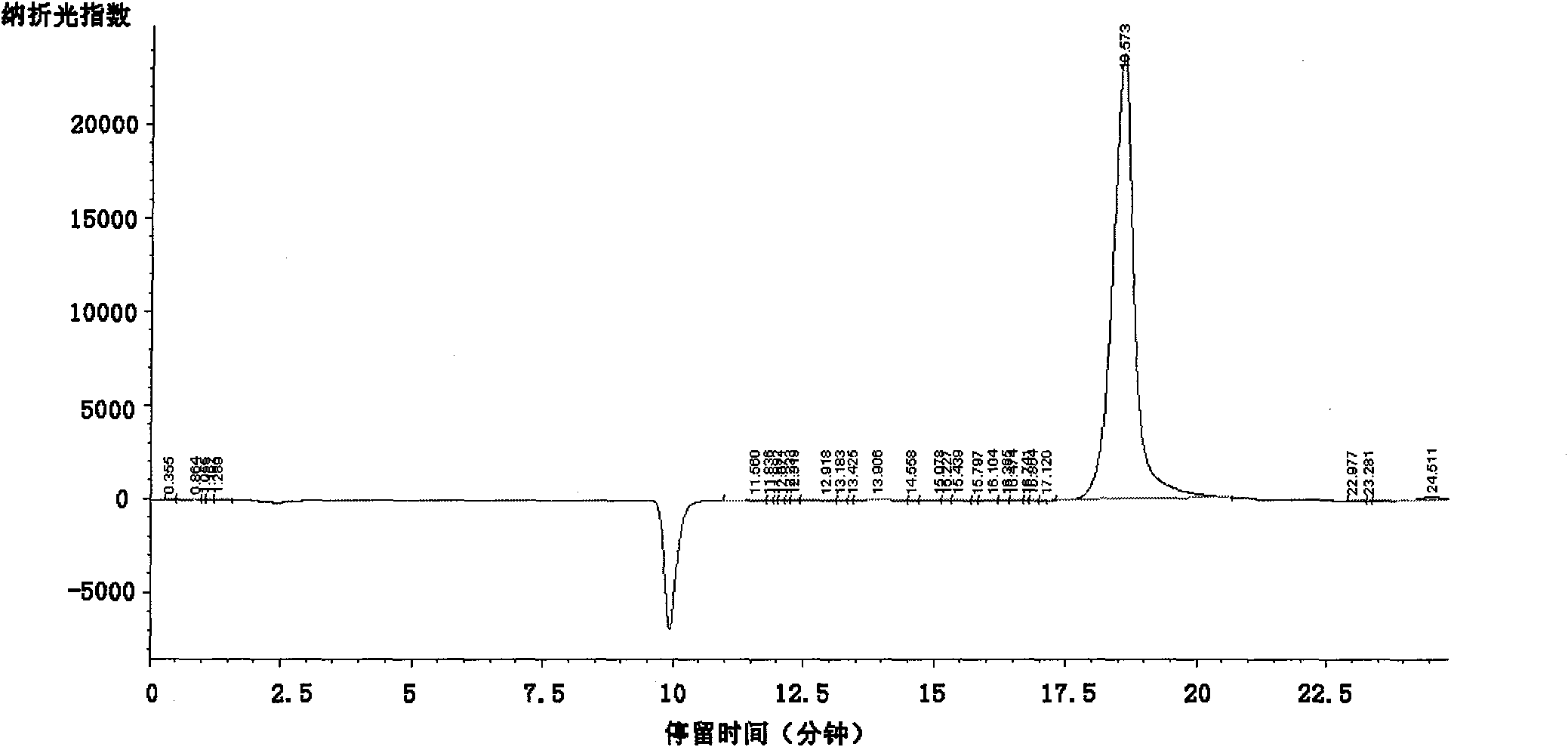
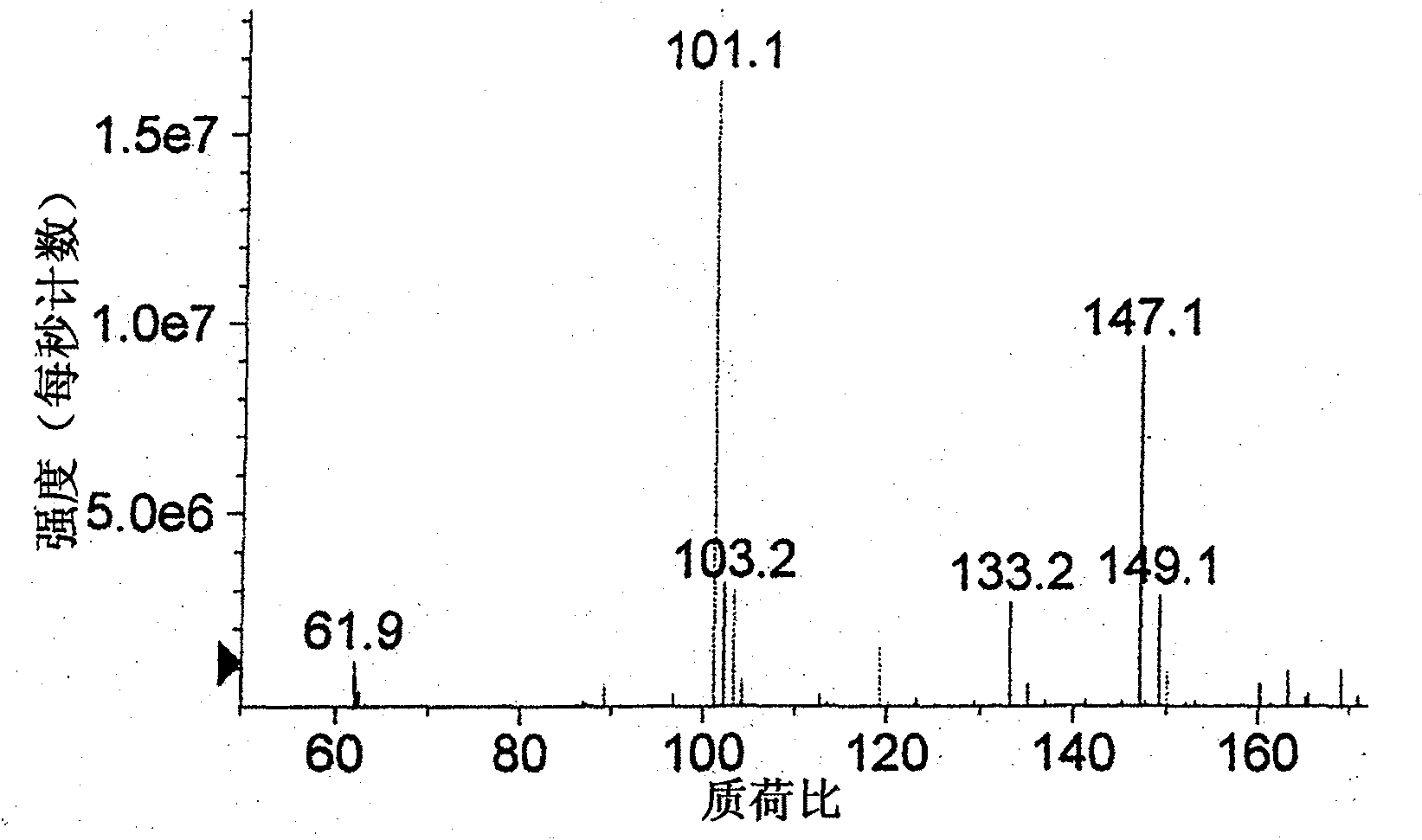
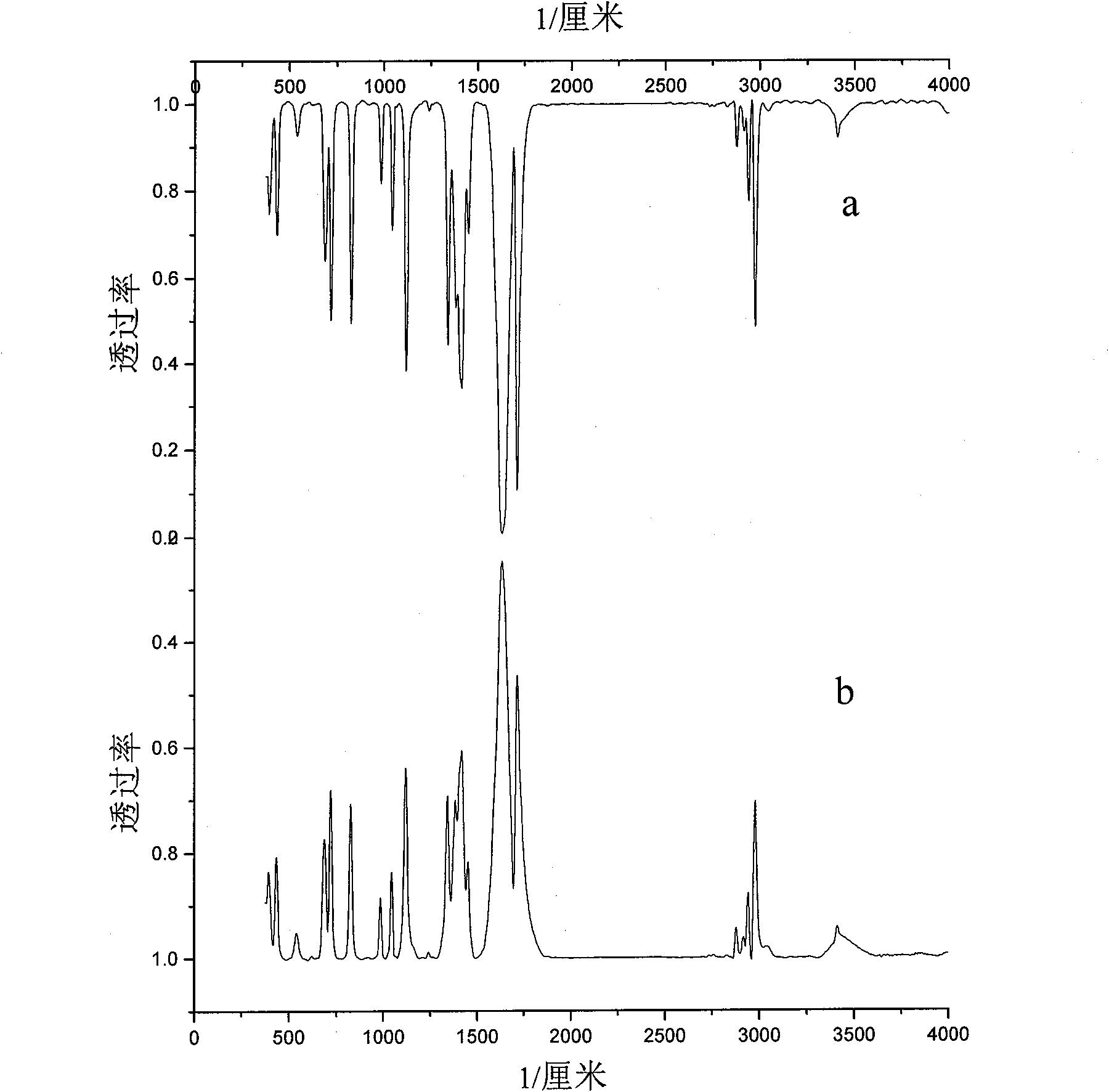
Method for preparing alpha-ketobutyric acid
A technology of ketobutyric acid and sodium ketobutyrate, applied in the direction of microorganism-based methods, biochemical equipment and methods, separation/purification of carboxylic acid compounds, etc., to achieve high conversion rate, convenient operation, and simple separation and extraction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Preparation of complete cell suspension containing α-hydroxyacid oxidase: Pseudomonas putida KT2440 (ATCC 47054) was selected for routine culture, during which 2,4-dinitrophenylhydrazine (DNP) and α-ketone The method of generating brown-red 2,4-dinitrophenylhydrazone by the action of butyric acid was used to detect the activity of α-hydroxyacid oxidase in cells, and when the activity of α-hydroxyacid oxidase reached 150 units / liter, the fermentation culture was terminated; separated and collected The thalline was washed 2 times with a pH 7.2 potassium phosphate buffer solution, and the thalline was resuspended in deionized water, so that the thalline concentration reached 200 grams of wet cells / liter, and a complete cell suspension containing α-hydroxyacid oxidase was obtained. Store at 4°C for later use;
[0040] (2) Transformation: the complete cell suspension prepared in step (1) is mixed with an aqueous solution of sodium α-hydroxybutyrate, and the concentration...
Embodiment 2
[0048] (1) Preparation of complete cell suspension containing α-hydroxyacid oxidase: Pseudomonas aeruginosa (ATCC15442) was selected for routine culture, during which 2,4-dinitrophenylhydrazine (DNP) and α-ketobutyl The method of generating brown-red 2,4-dinitrophenylhydrazone by the action of acid detects the activity of α-hydroxyacid oxidase in cells, and when the activity of α-hydroxyacid oxidase reaches 160 units / liter, stop the fermentation culture; separate and collect the bacteria , wash the cells twice with pH 7.2 potassium phosphate buffer, resuspend the cells in deionized water, make the cell concentration reach 200 g wet cells / liter, and obtain a complete cell suspension containing α-hydroxyacid oxidase, at 4°C store for later use;
[0049] (2) Transformation: The complete cell suspension prepared in step (1) is mixed with an aqueous solution of sodium α-hydroxybutyrate, and the concentration of sodium α-hydroxybutyrate in the mixture is 200 mmol / L, the concentratio...
Embodiment 3
[0057] (1) Preparation of complete cell suspension containing α-hydroxyacid oxidase: select Pseudomonas stutzeri A1501 (CGMCC0351), and conduct routine culture, during which 2,4-dinitrophenylhydrazine (DNP) and α-ketone The method of generating brown-red 2,4-dinitrophenylhydrazone by the action of butyric acid was used to detect the activity of α-hydroxyacid oxidase in cells, and when the activity of α-hydroxyacid oxidase reached 160 units / liter, the fermentation culture was terminated; separated and collected The thalline was washed 2 times with a pH 7.2 potassium phosphate buffer solution, and the thalline was resuspended in deionized water, so that the thalline concentration reached 200 grams of wet cells / liter, and a complete cell suspension containing α-hydroxyacid oxidase was obtained. Store at 4°C for later use;
[0058] (2) Transformation: The complete cell suspension prepared in step (1) is mixed with an aqueous solution of sodium α-hydroxybutyrate, and the concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com