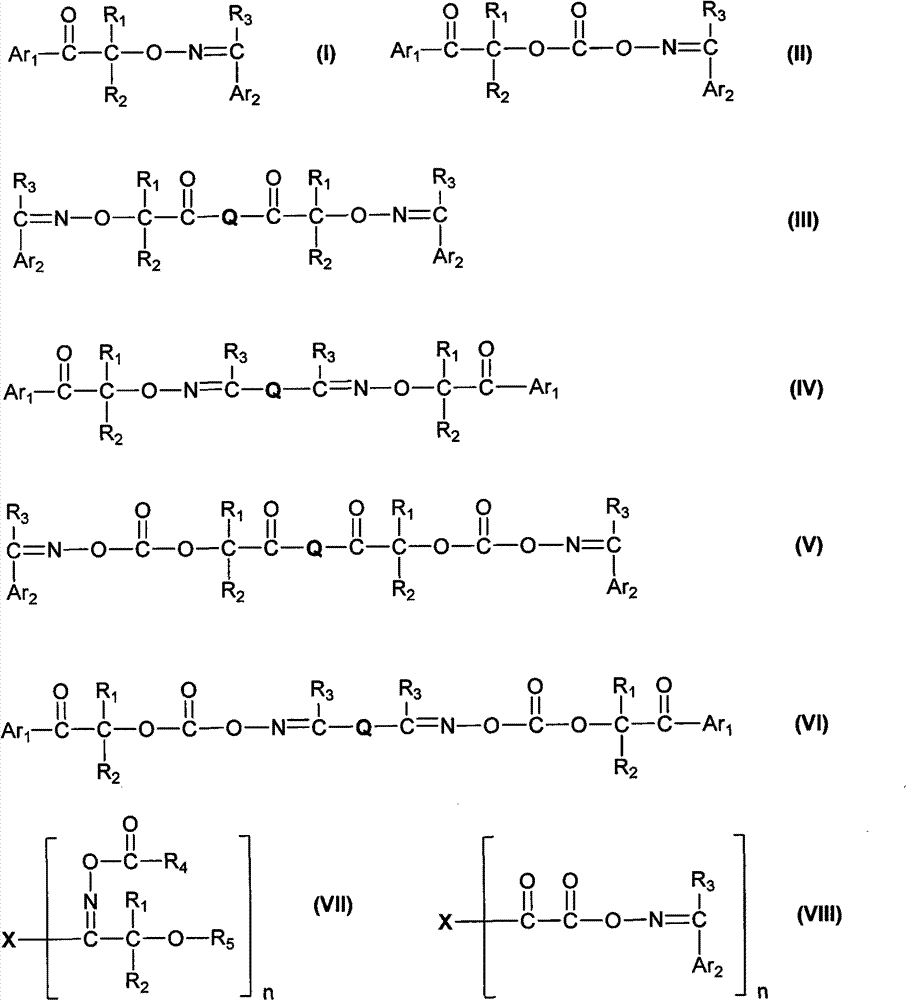
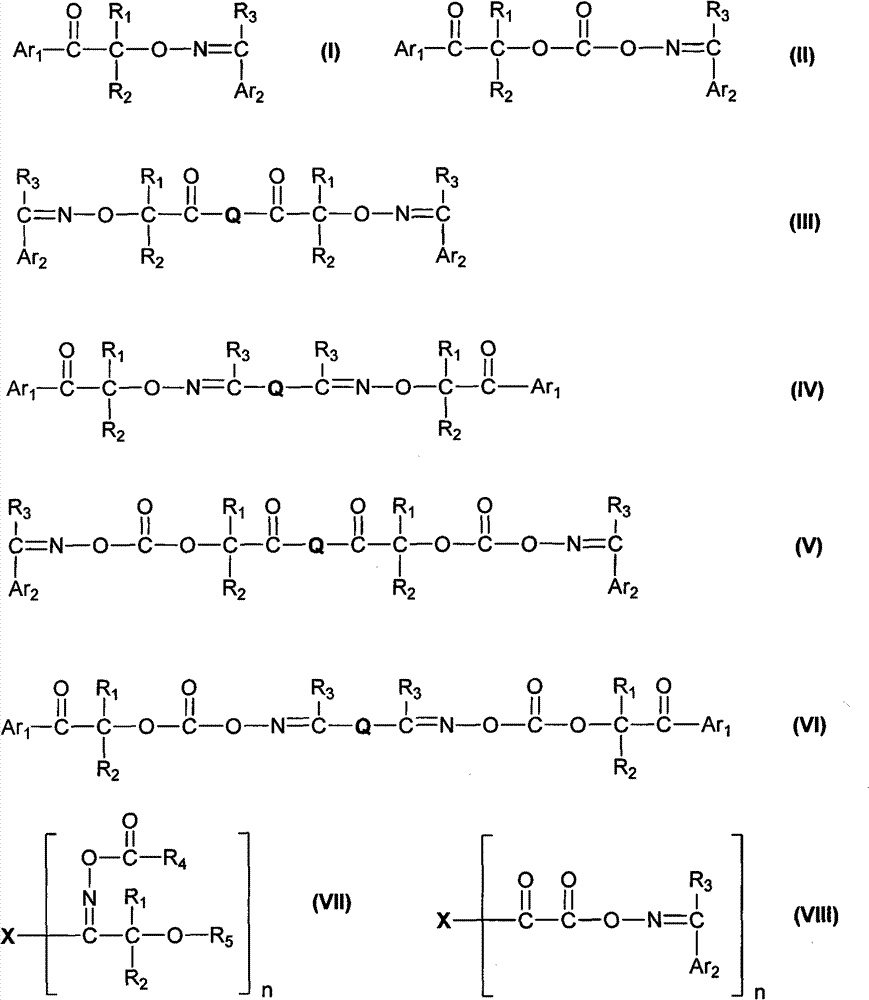
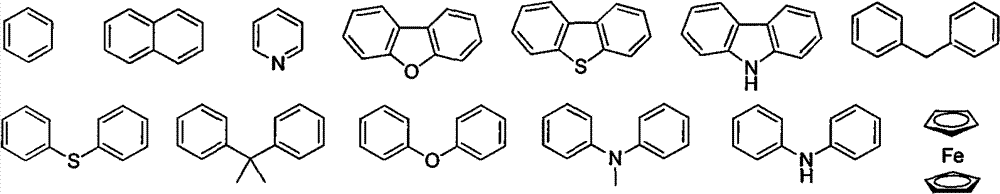
Aromatic ketone oxime photoinitiator compound
A technology of photoinitiator and compound, which is applied in the field of photoinitiator for free radical polymerization of ethylenically unsaturated system, can solve the problem that the new type of compound has not yet been reported, and achieves the effects of high initiation activity, easy preparation and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: (E)-2-Methyl-1-phenyl-2-(((1-phenylethylen)amine)oxy)-propan-1-one:
[0054]
[0055] Under the protection of nitrogen, put 7.3 g of (E)-acetophenone oxime in 120 ml of dry DMSO, cool to zero, and slowly add 2.6 g of NaH (60% mineral oil dispersed powder) under stirring. After half an hour, 14.6 g of 2-bromo-2-methyl-1-phenylpropan-1-one was added in batches, and after the addition, the reaction was stirred for 1 hour, and the temperature was raised to room temperature and reacted for 2 hours. The reaction solution was slowly poured into 300 ml of water, extracted with equal volume of ethyl acetate twice, the organic phases were combined, washed with saturated brine twice, dried with anhydrous sodium sulfate, filtered, and the solvent was evaporated. The residue was purified by silica gel column chromatography. Alkane was used as the eluent to obtain the target compound (12.6 g, 83% yield) as a pale yellow oily liquid, which solidified slowly when placed.
[0056...
Embodiment 2
[0064] Example 2: (E)-2-Methyl-1-phenyl-2-(((((1-phenylethylen)amine)oxy)carbonyl)oxy)-propan-1-one:
[0065]
[0066] Under the protection of nitrogen, mix 11.3 g Irgacure 1173, 15 ml dry pyridine, and 130 ml chloroform, cool to zero, and slowly add 29.1 g (Cl 3 CO) 2 C=O, after the addition, stir and react for half an hour, then warm to room temperature and react for 2 hours. 11.2 g of (E)-acetophenone oxime was added to the system in batches, 9 ml of pyridine was added, and the reaction was continued for 8 hours. The end of the reaction was monitored by thin-layer chromatography. The reaction solution was slowly poured into 250 ml of water, diluted with 80 ml of chloroform, shaken to separate the organic phase, washed twice with saturated brine, dried over anhydrous sodium sulfate, filtered, and evaporated to remove the solvent. The residue was purified on silica gel column chromatography to 9: 1 hexane / ethyl acetate was used as the eluent to obtain the target compound (14.1 g...
Embodiment 3
[0073] Example 3: (E)-1,1'-(thiobis(4,1-phenylene))bis(2-methyl-2-(((E)-(1-phenylethylene)) Amine) Oxygen) Propan-1-one:
[0074]
[0075] Under the protection of nitrogen, place 10.1 g of (E)-acetophenone oxime in 180 ml of dry DMSO, cool to zero, and slowly add 3.6 g of NaH (60% mineral oil dispersed powder) with stirring. After 1 hour, Add 18.1 g of 1,1'-(thiobis(4,1-phenylene))bis(2-bromo-2-methylpropane-1-one) in batches, stir and react for 2 hours after the addition, and warm to room temperature React for another 4 hours. The reaction solution was slowly poured into 500 ml of water, extracted with equal volume of ethyl acetate twice, the organic phases were combined, washed with saturated brine twice, dried with anhydrous sodium sulfate, filtered, and the solvent was evaporated. The residue was purified on silica gel column chromatography with 12 :1 hexane / ethyl acetate as the eluent to obtain the target compound (11.7 g, 53% yield) as a yellow oily liquid, which slowly s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com