Aromatic Ketophosphine Oxy Hybrid Compounds
A compound, phosphine oxide technology, applied in the field of aromatic ketophosphine oxide hybrid compounds, can solve the problems of rarity, no disclosure or mention of compound properties or uses, and achieve the effects of easy operation, high initiation activity, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
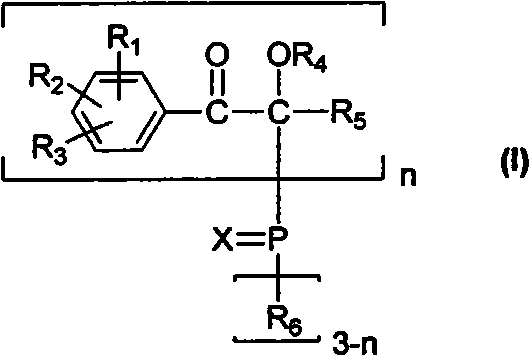
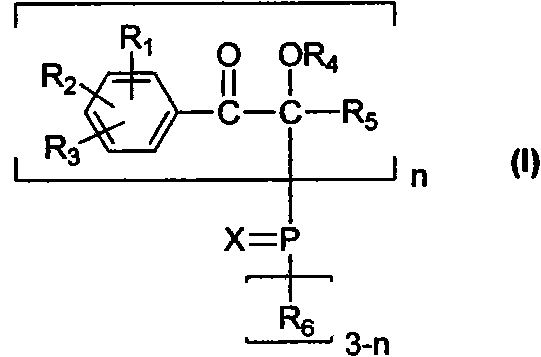
[0017] The present invention discloses the preparation method of the compound of general formula (I) at the same time, these methods rely on some new reaction schemes, and as far as each unit reaction involved in the scheme is well known to those skilled in the art, it can be conveniently use.
[0018] When n takes a value of 1, the general reaction formula is as follows:
[0019]
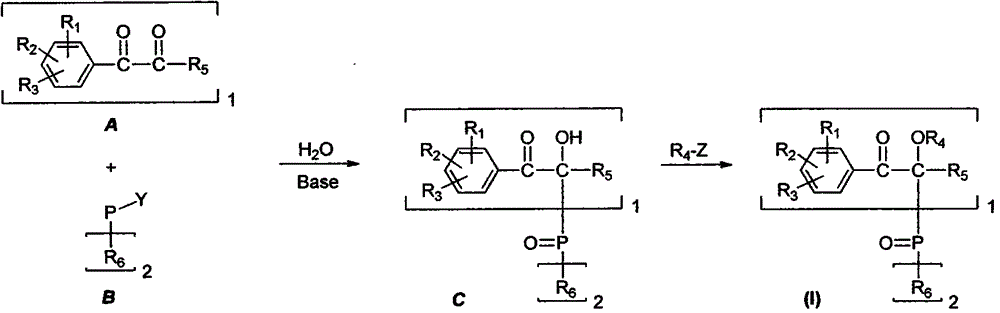
[0020] The general formula of diketone precursor A and two molecules is YP of B (R 6 ) 2 [Y is H or Cl] carry out alkali-promoted condensation to obtain intermediate C, and the appropriate base can be inorganic hydroxide, alkali metal, alkoxy metal salt, or organic amine compound (aryl amine or alkyl amine). When Y is chlorine, H 2 The participation of O is necessary and the P atom in the generated C is directly a pentavalent phosphine oxide; when Y is hydrogen, it needs to be oxidized to obtain a pentavalent phosphine oxide unit after condensation. Suitable oxidants include hydrogen peroxide...
Embodiment 1
[0037]
[0038] At room temperature, take 2.1 grams of dibenzoyl and place it in a mixture of 30 milliliters of 1,4-dioxane and water (volume ratio 1:4), stir until completely dissolved, then slowly add 2.2 milliliters of dibenzoyl Phenylphosphonium chloride. After the dropwise addition was completed, the temperature was raised to 80° C. and stirring was continued for 2 hours (TLC tracking). After the reaction was completed, it was extracted three times with 30 ml of ethyl acetate, the organic phases were combined, concentrated by rotary evaporation and dried to obtain about 4.0 g of the target compound as a white solid, with a yield of about 96%.
[0039] H NMR spectrum 1 H NMR (CDCl 3): 7.88-7.81(m, 4H), 7.76-7.69(m, 2H), 7.49-7.24(m, 13H), 6.74(d, 1H, J=9.6Hz); C NMR 13 C NMR (CDCl 3 ): 193.9, 135.0, 134.9, 134.3, 133.3, 132.3, 132.1, 131.7, 131.6, 130.3, 128.9, 128.8, 128.5, 128.3, 128.2, 77.3ppm;
[0040] Elemental analysis (molecular formula C 26 h 21 PO 3 ): ...
Embodiment 2
[0043]
[0044] Under the protection of nitrogen, put 2.8 g of the product of Example 1 above into 50 ml of dry DMF, add 1.2 g of iodomethane and 1.9 g of silver oxide powder in sequence, heat at 50°C for 2 hours, then return to room temperature and stir overnight. The reaction solution was filtered with diatomaceous earth, poured into 200 ml of water, extracted 3 times with 150 ml of ethyl acetate, combined the organic phases, washed 2 times with saturated brine, dried over anhydrous sodium sulfate, filtered, evaporated to remove the solvent, and the residue was in The title compound (1.1 g, 37% yield) was obtained by silica gel column chromatography with hexane / ethyl acetate as eluent.
[0045] Elemental analysis (molecular formula C 27 h 23 PO 3 ):
[0046] Theoretical: Carbon, 76.04%; Hydrogen, 5.44%; Found: Carbon, 75.97%; Hydrogen, 5.39%.
[0047] HRMS high resolution mass spectrometry [molecular formula M+Na: C 27 h 23 PO 3 Na]:
[0048] Theoretical value: 44...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com