Vaccine adjuvant of swine mycoplasmal pneumonia live vaccine, and preparation method and application thereof
A technology for mycoplasma pneumonia and vaccine adjuvants, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, bacterial antigen components, etc., can solve the high requirements of stability and preparation process, and the water-in-oil adjuvants are not Application and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
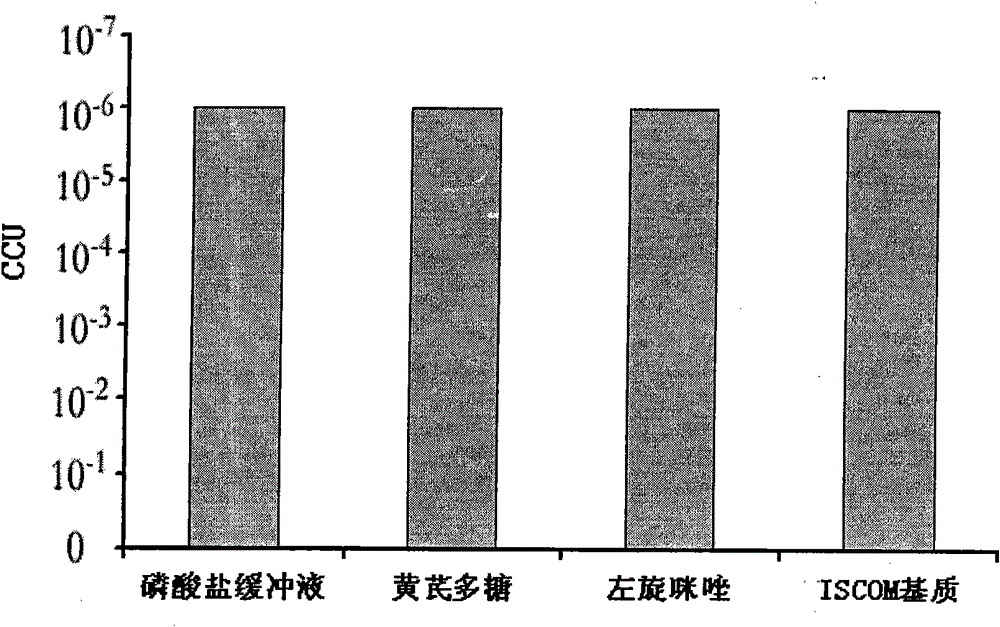
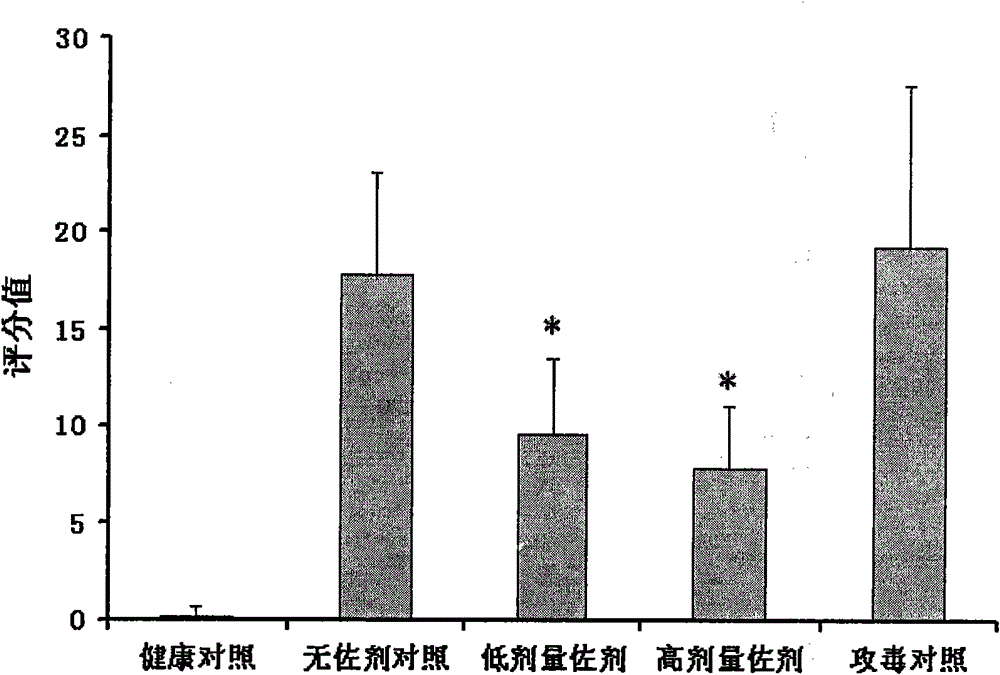
[0025] Example 1 Toxicity Detection of Different Adjuvant Components to Live Vaccine of Mycoplasma Porcine Pneumonia
[0026] 1. Experimental materials
[0027] 1. Vaccine: Mycoplasma swine pneumonia (168 strains) live vaccine, provided by Nanjing Tianbang Biotechnology Co., Ltd.;
[0028] 2. Adjuvant ingredients: astragalus polysaccharide (purchased from Shaanxi Jintai Bioengineering Co., Ltd.); levamisole hydrochloride injection (purchased from Jiangsu Huadong Bell Biopharmaceutical Co., Ltd.); ISCOM matrix (made in the laboratory).
[0029] 2. Experimental method:
[0030] Various different adjuvant components were prepared into solutions of certain concentrations with phosphate buffer solution, and the concentrations of various adjuvant components were as follows:
[0031] Astragalus polysaccharide: 200mg / ml
[0032] Levamisole: 25mg / ml
[0033] ISCOM matrix: 5mg / ml (concentration of Quil A component contained)
[0034] Above-mentioned three kinds of adjuvant solution...
Embodiment 2
[0037]Embodiment 2 is applicable to the adjuvant and preparation thereof of porcine mycoplasma pneumonia live vaccine
[0038] The vaccine adjuvant of the swine mycoplasma pneumonia live vaccine of the present invention is characterized in that the adjuvant solution contains ISCOM matrix, levamisole, Chinese herbal medicine polysaccharide and phosphate buffer solution, and each ml solution of the adjuvant contains: ISCOM matrix 0.1-2mg of QuilA, 2-10mg of levamisole, 20-100mg of Chinese herbal polysaccharide, and phosphate buffer saline for the rest.
[0039] The above-mentioned Chinese herbal medicine polysaccharide is selected from lentinan or astragalus polysaccharide or wolfberry polysaccharide or privet fruit polysaccharide.
[0040] The preparation method of the vaccine adjuvant of the present invention comprises: first preparing a high-concentration mother solution, then preparing a low-concentration adjuvant solution, and finally diluting it to the required concentrati...
Embodiment 3
[0060] The preparation of embodiment 3 adjuvanted mycoplasma pneumonia live vaccine
[0061] Complete the vaccine adjuvant prepared by the method described in Example 2, the volume ratio of live vaccine and vaccine adjuvant is 1: 1~1: 5 according to the ratio of preparing and mixing to be the adjuvanted Mycoplasma suis Pneumonia Live Vaccine. The preferred volume ratio of the live vaccine against Mycoplasma pneumoniae of swine to the vaccine adjuvant is 1:2-1:3. After being mixed, the live vaccine of mycoplasma pneumoniae containing adjuvant can be improved from intrapulmonary injection immunity to intramuscular injection immunity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com