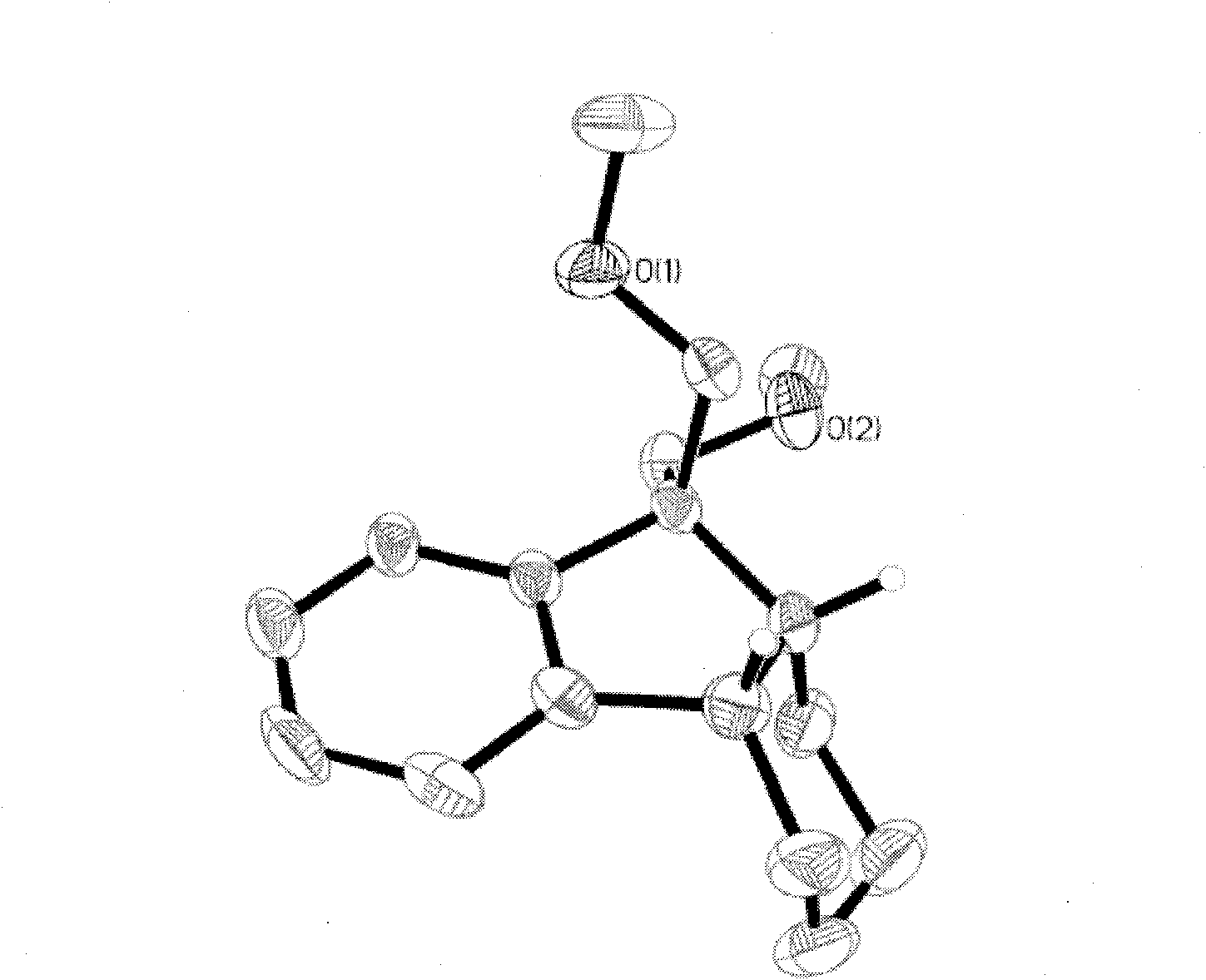
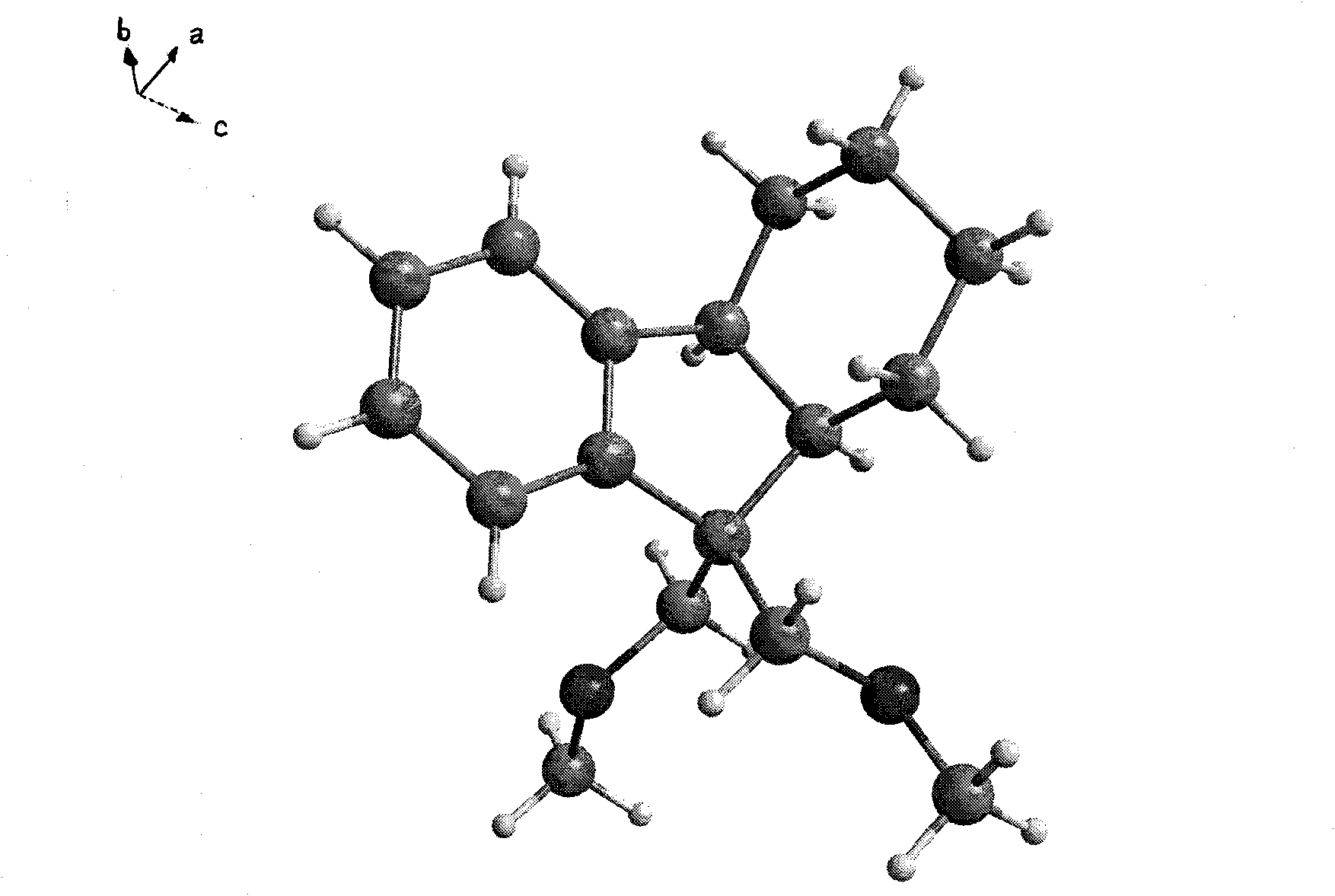
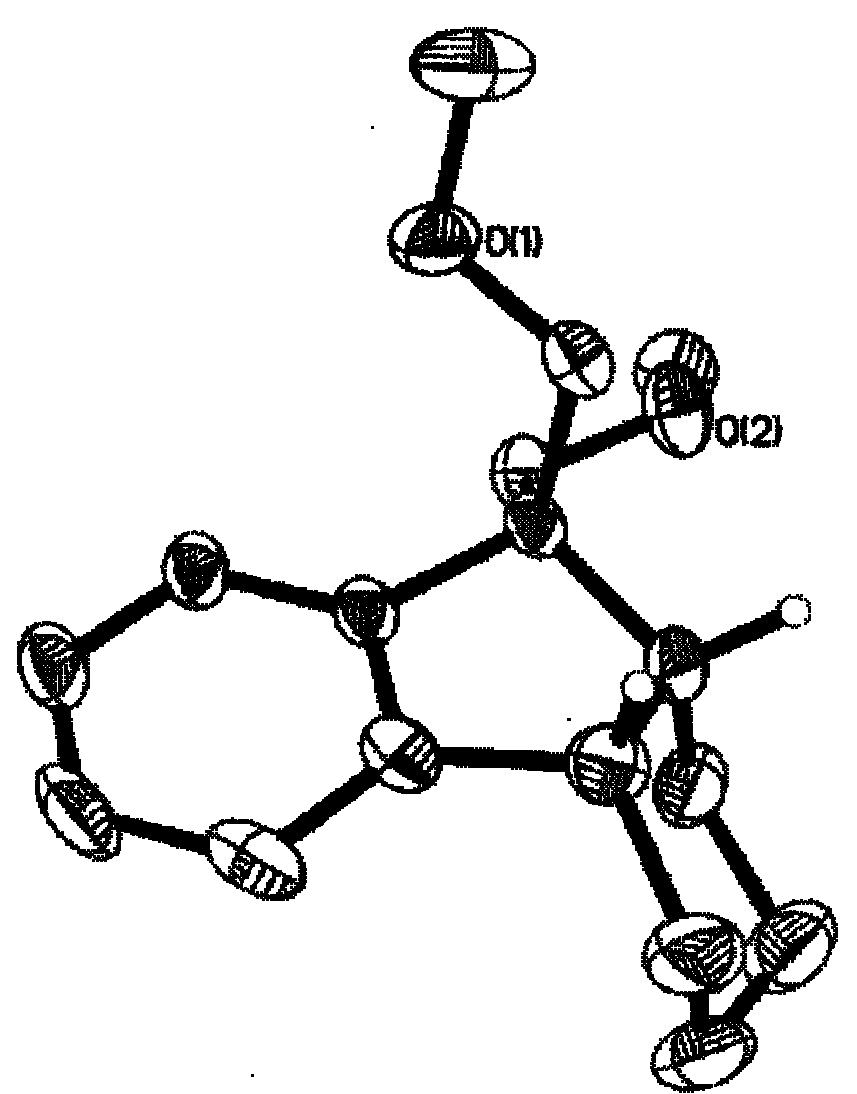
Method for selective catalytic hydrogenation for 9,9-bi(methoxymethylated) fluorine (BMMF)
A methoxymethyl, catalytic hydrogenation technology, applied in 9 fields, achieves the effects of good repeatability, simple operation and high product stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In an autoclave with a volume of 75 milliliters, 2.03 grams of BMMF, 20 milliliters of cyclohexane, and 0.25 grams of activated modified skeleton nickel catalyst were put into the autoclave. MPa of hydrogen, put it into an oil bath and slowly heat up to 120°C, adjust the hydrogen valve to make the system pressure reach 2.0MPa, react for 320min, analyze it by gas chromatography after cooling, the conversion rate of raw materials is 100%, and the product cis-H 6 The selectivity of BMMF was 96.6%.
Embodiment 2
[0023] In an autoclave with a volume of 75 milliliters, 2.03 grams of BMMF, 20 milliliters of tetrahydrofuran, and 0.25 grams of activated modified skeleton nickel catalyst were put into the autoclave. Put hydrogen in an oil bath and slowly heat up to 140°C, adjust the hydrogen valve to make the system pressure reach 2.0MPa, react for 30min, and analyze by gas chromatography after cooling, the conversion rate of raw materials is 100%, and the product cis-H 6 The selectivity of BMMF was 96.1%.
Embodiment 3
[0025] In an autoclave with a volume of 75 milliliters, 2.03 grams of BMMF, 20 milliliters of n-hexane, and 0.25 grams of activated modified skeleton nickel catalyst were put into the autoclave, and the reactor was closed, replaced with nitrogen for 3 times, replaced with hydrogen for 3 times, and then filled with 0.5MPa Put the hydrogen in an oil bath and slowly heat up to 130°C, adjust the hydrogen valve to make the system pressure reach 2.0MPa, react for 60min, and analyze it by gas chromatography after cooling. The conversion rate of raw materials is 100%, and the product cis-H 6 The selectivity of BMMF was 96.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com