Method for testing content of pinoresinol diglucoside in compound eucommia bark tablet
A technology of pinoresinol diglucoside and compound eucommia tablet is applied in the directions of measuring devices, medical preparations containing active ingredients, pharmaceutical formulations, etc., and can solve the problem of inability to separate pinoresinol diglucoside, difficult filtration, and undetectable components, etc. To increase the content determination method, control the quality, and ensure the effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
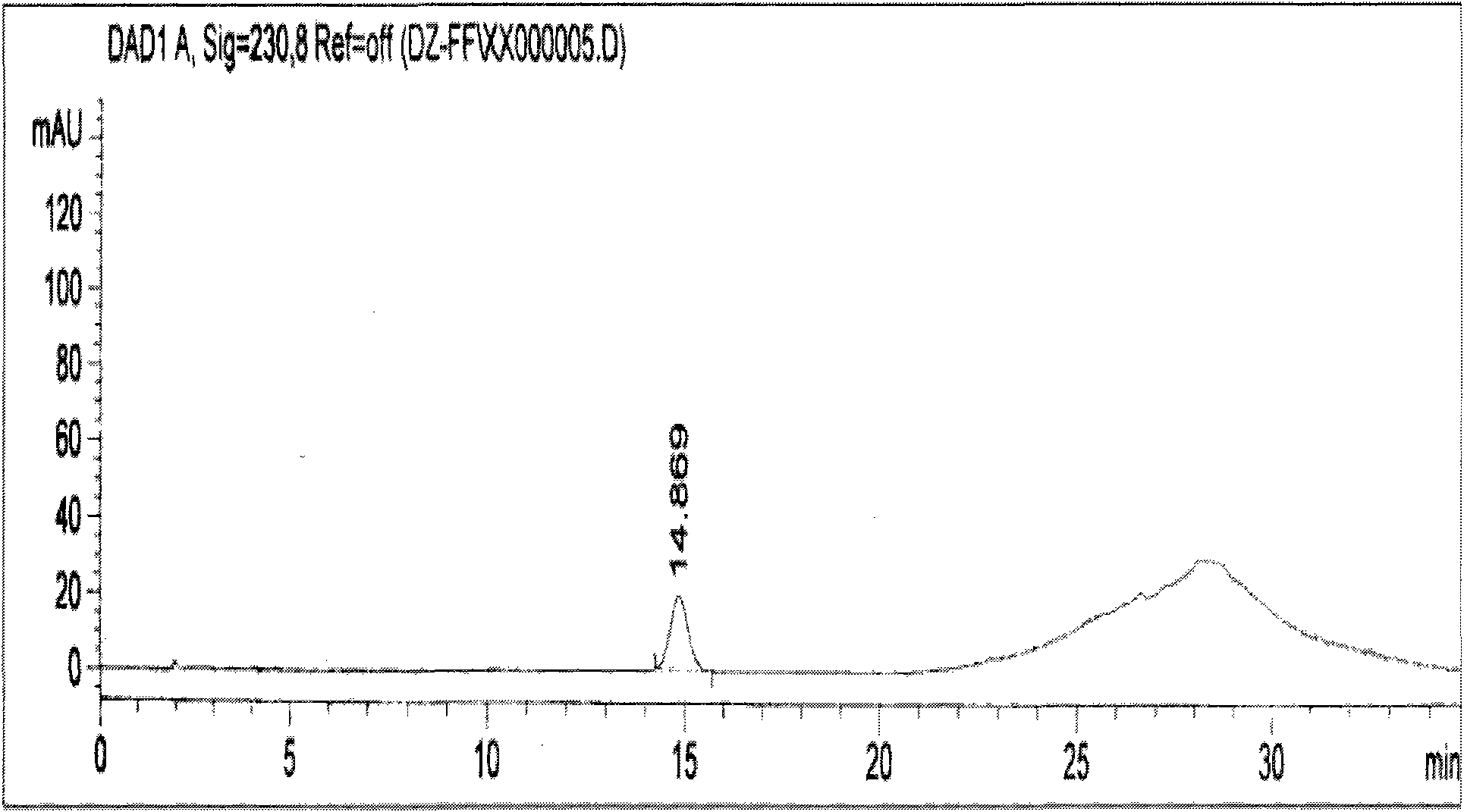
[0033] Determination of the content detection method of pinoresinol diglucoside in the compound eucommia ulmoides tablet of embodiment 1
[0034] The chromatographic conditions use octadecylsilane and silica gel as filler; methanol as mobile phase A and water as mobile phase B for gradient elution; detection wavelength: 230nm; flow rate: 1ml / min; theoretical The number of plates should not be less than 2000 based on the peak of pinoresinol diglucoside; the gradient elution should be carried out according to the following procedure:
[0035]
[0036] Preparation of reference substance solution Accurately weigh 14.8mg of pinoresinol diglucoside reference substance, put it in a 50ml measuring bottle, add 30% methanol to dissolve and set the volume to the mark, and obtain a 0.296mg / ml reference substance solution.
[0037] Preparation of the test solution Take compound Eucommia tablets, remove the coating, grind finely, accurately weigh 1g of the fine powder, put it in a conica...
Embodiment 2
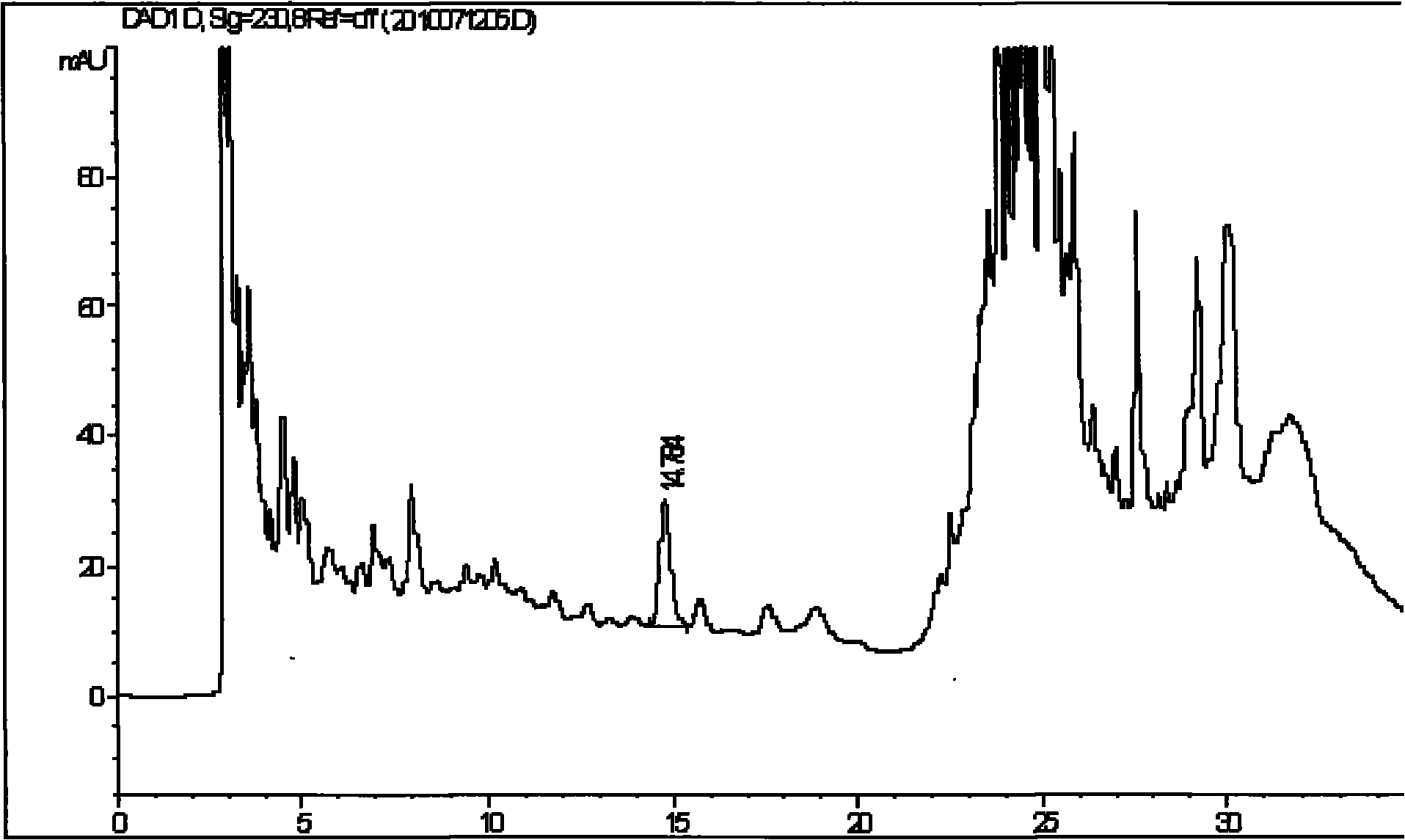
[0056] Embodiment 2 Get the batch number 200701001 batch of compound Eucommia tablets produced by Guangzhou Xiangxue Pharmaceutical Co., Ltd., the sheet weight is 0.35 grams, and detect according to the following conditions:
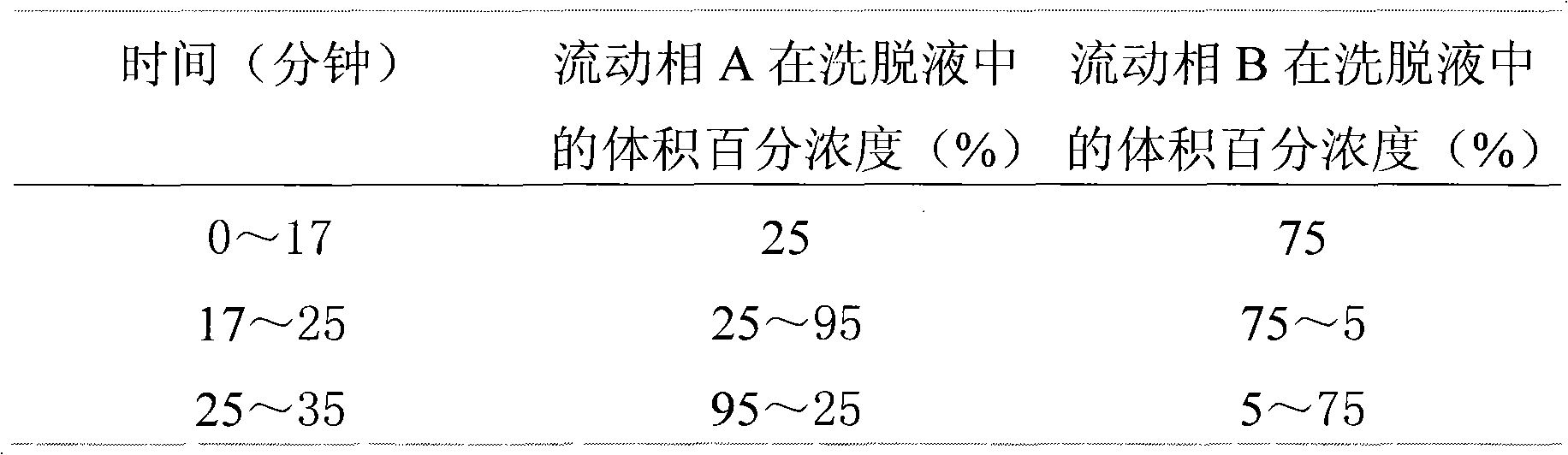
[0057] (1) Chromatographic conditions: Carbooctadecylsilane and silica gel are used as filler; methanol is used as mobile phase A, and water is used as mobile phase B to carry out gradient elution with eluent composed of mobile phase B. The elution procedure is shown in Table 4; Wavelength: 230nm; flow rate: 1ml / min; the number of theoretical plates calculated based on pinoresinol diglucoside peak should not be less than 2000.
[0058] Table 4
[0059]
[0060] (2) Preparation of the reference substance solution: Accurately weigh the reference substance of pinoresinol diglucoside, add a methanol solution with a concentration of 25% by volume to prepare a solution containing 0.035 mg per 1 ml, and obtain the product.
[0061] (3) Preparation of the te...
Embodiment 3
[0064] Embodiment 3 Measure other batches of products produced by Guangzhou Xiangxue Pharmaceutical according to the method in Example 2, and the measurement results are as follows:
[0065]
[0066]
[0067] From the results of the above batches of determination, it can be seen that the content of pinoresinol diglucoside in the compound eucommia tablets is greater than 0.05 mg per tablet, and the weight of each substrate is 0.35 g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com