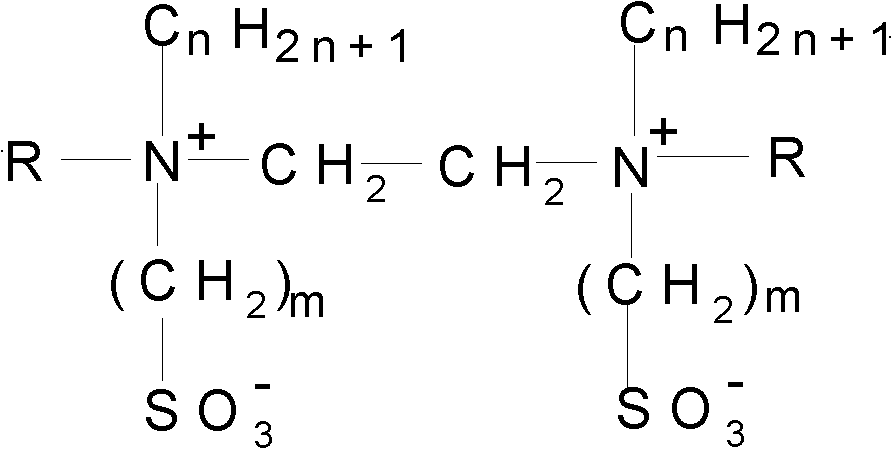Sulphobetaine ampholytic gemini surfactant and synthesis method thereof
A technology of sulfobetaine type and sulfobetaine, which is applied in the field of amphoteric gemini surfactants, can solve the problems of less research on sulfobetaine type gemini surfactants and limitations in the application of surfactants, and achieve easy separation, Excellent surface properties and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、1
[0012] Embodiment 1, the synthesis of 1,2-two [N-methyl-N-(4-sulfobutyl)-tetradecyl ammonium] ethyl betaine gemini amphoteric surfactant: one, intermediate (N, The synthesis of N'-(two)tetradecyl-N, N'-dimethylethylenediamine): in a 250ml four-necked reaction flask equipped with a reflux condenser, a magnetic stirrer, a constant pressure dropping funnel, and a thermometer Add 0.2mol (8.00g) of sodium hydroxide and 100ml of absolute ethanol to the solution, after completely dissolving at 45°C, add 0.1mol (8.80g) of N,N'-dimethylethylenediamine to the reactor, and dissolve 0.2mol of (55.46g) bromotetradecane was added dropwise to the system and then heated to reflux for 48h. After the reaction, the system was cooled to room temperature, filtered to remove the solid, heated to 100°C for rotary evaporation under reduced pressure, filtered, added an appropriate amount of absolute ethanol, and repeated the above operation until no solid was precipitated to obtain the intermediate N,...
Embodiment 2
[0013] Example 2, the structural characterization of the target product: Table 1 GS14-4 1 H NMR spectrum analysis The above results indicated that the structure of the synthesized target product was consistent with the designed structure.
Embodiment 3
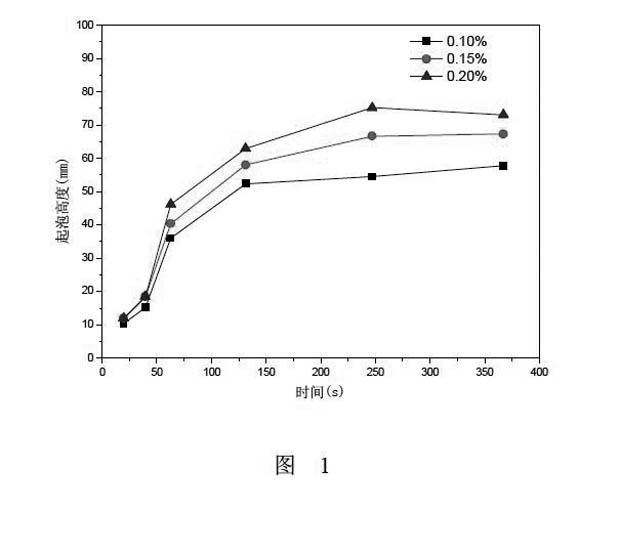
[0014] Example 3, Ross-Miles determination of GS 14-4 foam performance test: the test method is at a temperature of (45 ± 0.5) ° C, 200mL sample solution is flowed down from a pore with a height of 900mm and an inner diameter of 2.9mm, and washed Into 50mL sample solution with the same temperature and the same concentration, write down the foam height when the 200mL solution has flowed out as the evaluation index of the foaming ability of the tested sample, and the foam height at different moments after foaming is used as the evaluation index of foam stability. See the experimental results figure 1 .
[0015] Experiment is carried out to the foaming performance of amphoteric gemini surfactant GS 14-4 in embodiment 1, the result is as follows figure 1 As shown, as the concentration of GS 14-4 increases, the foam height gradually increases, but when the concentration is greater than 0.15%, the foam height hardly increases, indicating that the developed GS 14-4 has an average co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com