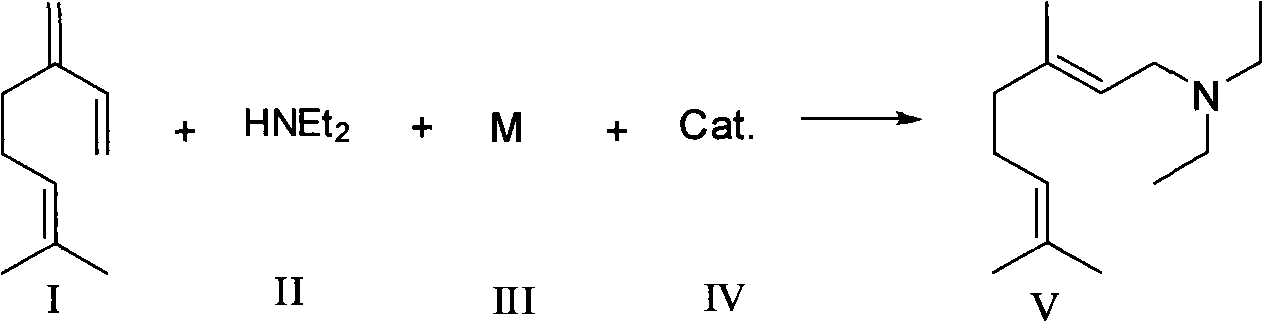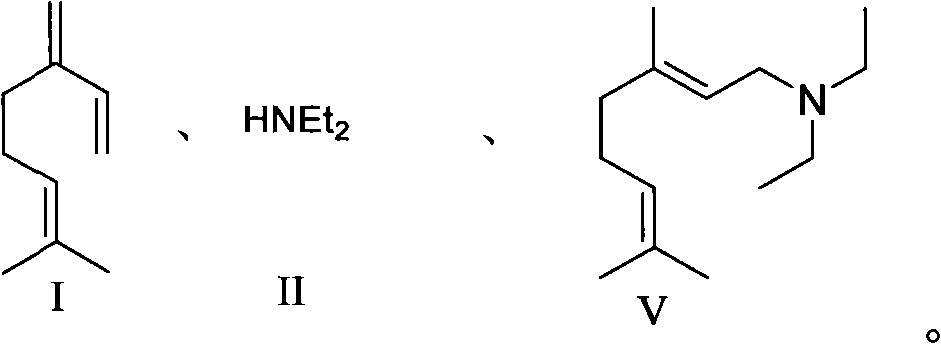Synthesizing method of N,N-diethyl-3,7-dimethyl-(E)-2,6-octadiene-1-amine
A technology of dimethyl and octadiene, which is applied in chemical instruments and methods, condensation/addition reactions to prepare amino compounds, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of low yield, Not suitable for industrial production and other problems, to achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Under the protection of nitrogen, add 10ml toluene, 17g myrcene (purity 80%), 10g diethylamine, 0.2g lithium, 4g styrene to a 100ml reaction flask under the protection of nitrogen, heat to 40 degree, after stirring for 18 hours, stop heating. When the temperature drops to room temperature, slowly add 20ml of deionized water dropwise to keep the system temperature below 40°C. After the dropwise addition is complete, stir for another 30 minutes, let it stand for liquid separation, extract the water phase with 20ml of toluene twice, combine and add to the organic phase , toluene and some impurities were evaporated under reduced pressure to obtain 20.6 g of colorless liquid, GC showed N, N-diethyl-3,7-dimethyl-(E)-2,6-octadien-1-amine The content is 92%, and the yield is 90.7%.
Embodiment 2
[0012] Under the protection of nitrogen, add 15ml cyclohexane, 17g myrcene (80% purity), 10g diethylamine, 0.2g lithium, 5g naphthalene to a 100ml reaction flask, heat to 80 degrees, after stirring for 15 hours, stop heating . When the temperature drops to room temperature, slowly add 20ml of deionized water dropwise, keeping the system temperature not exceeding 40 degrees, after the dropwise addition is completed, stir for another 30min, let it stand for liquid separation, extract the water phase twice with 20ml cyclohexane, combine and add In the organic phase, cyclohexane and some impurities were evaporated under reduced pressure to obtain 21 g of a colorless liquid. GC showed that N, N-diethyl-3,7-dimethyl-(E)-2,6-octadiene- The content of 1-amine was 91%, and the yield was 91%.
Embodiment 3
[0014] Under the protection of nitrogen, add 15ml methyl tert-butyl ether, 10g diethylamine, 0.2g lithium, 0.1g sodium, 5g styrene to a 100ml reaction bottle, after stirring for 30min, add 17g myrcene (purity 80%) , sealed, heated to 60 degrees, and after stirring for 17 hours, stop heating. When the temperature drops to room temperature, slowly add 20ml of deionized water dropwise to keep the system temperature below 40°C. After the dropwise addition is complete, stir for another 30min, let it stand for liquid separation, and extract the water phase twice with 20ml of methyl tert-butyl ether , combined and added to the organic phase, and evaporated under reduced pressure to remove methyl tert-butyl ether and some impurities to obtain 20.7 g of a colorless liquid. GC showed that N, N-diethyl-3,7-dimethyl-(E)-2 , the content of 6-octadien-1-amine was 93%, and the yield was 92.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com