Method for measuring flavone glycoside compounds in herba epimedii
A technology for determination of flavonoid glycosides in A. chinensis, which is applied in the field of determination of Epimedium, can solve the problems of weak anti-interference ability, inaccurate quantification, and difficulty in baseline separation, and achieves the effects of good accuracy, high sensitivity, and easy baseline separation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] a. Weigh 0.5g of the Chinese herbal medicine Epimedium sample of 40 mesh sieves in a 100mL stoppered glass conical flask, add 50mL of 50% methanol-water solution (V / V), weigh, at normal temperature, under ultrasonic power Ultrasonic at 350W and ultrasonic frequency of 70KHz for 30min to make up the weight.
[0016] b. Take the supernatant and filter it with a 0.22um organic phase membrane to obtain the sample to be analyzed.
[0017] c. Analyze the obtained sample to be analyzed:
[0018] 1. Chromatographic conditions: Analytical column: Waters Acquity UPLC BEH C 18 (1.7μm, 2.1mm×50mm); mobile phase: phase A is 0.1% formic acid-water solution (V / V), phase B is acetonitrile solution; gradient condition: 0min, A: B (90%: 10%); 10min, A:B (80%:20%); 25min, A:B (70%:30%); 35min, A:B (50%:50%); 35.1min, A:B (0%:100 %); 39.9 min, A:B (0%:100%) 40.0 min, A:B (90%:10%). Flow rate: 0.25mL / min; column temperature 30°C.
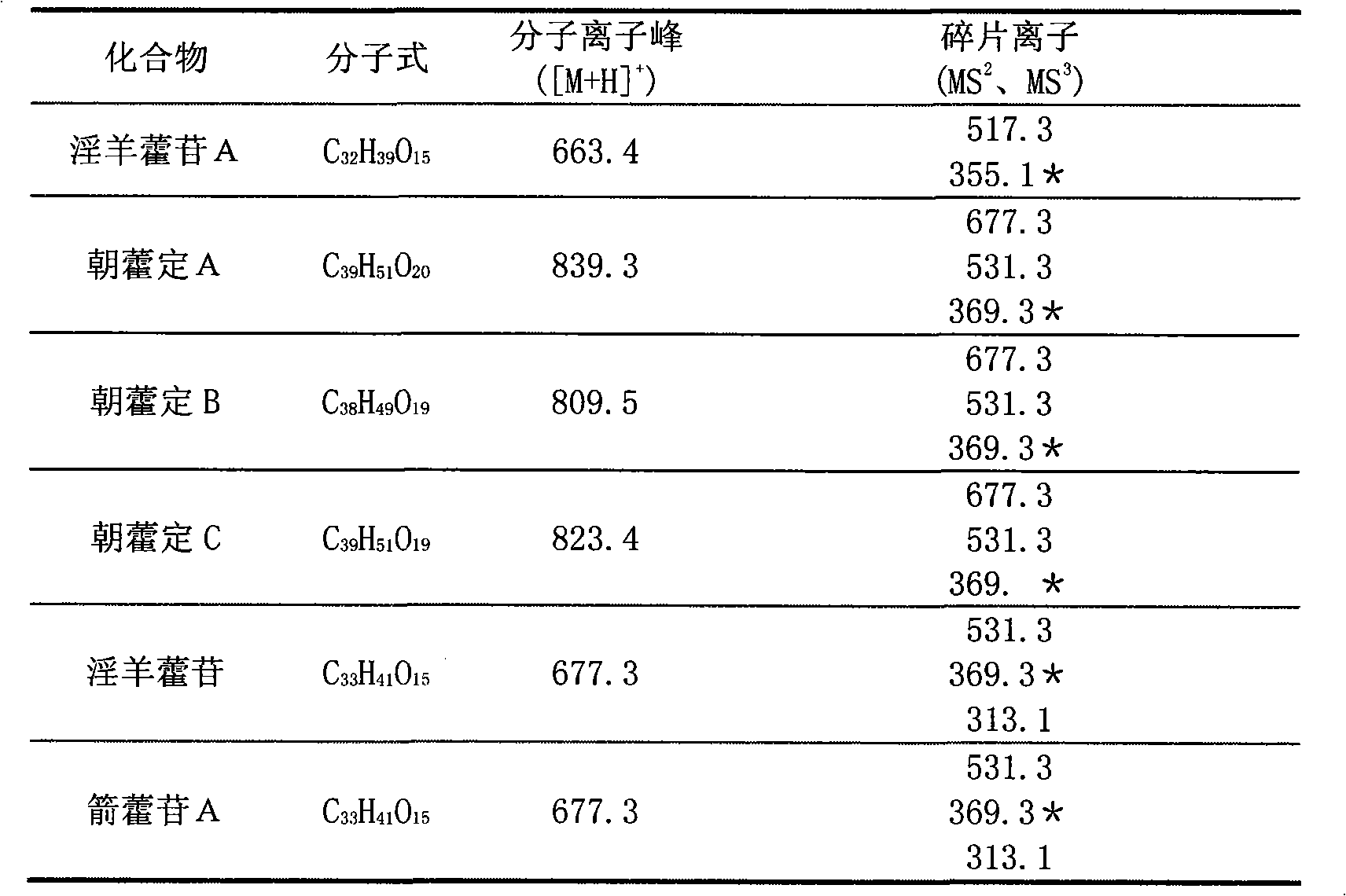
[0019] 2. Mass spectrometry conditions: Quantitative a...
Embodiment 2
[0024] a. Take by weighing 0.4g of the Chinese herbal medicine Epimedium sample of 60 mesh sieves in a 100mL stoppered glass conical flask, add 40mL of 60% methanol-water solution (V / V), weigh, under normal temperature, at a rotating speed of 150r / Shake for 30 minutes at 2 min, let stand for 5 minutes, and make up the weight.
[0025] b. Take the supernatant and filter it with a 0.22um organic phase membrane to obtain the sample to be analyzed.
[0026] c. Analyze the obtained sample to be analyzed:
[0027] 1. Chromatographic conditions: Analytical column: Waters Acquity UPLC BEH C 18 (1.7μm, 2.1mm×50mm); mobile phase: phase A is 0.2% formic acid-water solution (V / V), phase B is acetonitrile solution; gradient condition: 0min, A: B (90%: 10%); 10min, A:B (80%:20%); 25min, A:B (70%:30%); 35min, A:B (50%:50%); 35.1min, A:B (0%:100 %); 39.9 min, A:B (0%:100%) 40.0 min, A:B (90%:10%). Flow rate: 0.25mL / min; column temperature 30°C.
[0028] 2. Mass spectrometry conditions: ...
Embodiment 3
[0030] a. Take by weighing 0.5 g of the Chinese herbal medicine Epimedium sample of 80 mesh sieves in a 100 mL glass conical flask with a stopper, add 20 mL of 70% methanol-water solution (V / V), weigh, and soak for 1.5 h at normal temperature, Make up weight.
[0031] b. Take the supernatant and filter it with a 0.22um organic phase membrane to obtain the sample to be analyzed.
[0032] c. Analyze the obtained sample to be analyzed:
[0033] 1. Chromatographic conditions: Analytical column: Waters Acquity UPLC BEH C 18 (1.7μm, 2.1mm×50mm); mobile phase: phase A is 0.3% formic acid-water solution (V / V), phase B is acetonitrile solution; gradient condition: 0min, A: B (90%: 10%); 10min, A:B (80%:20%); 25min, A:B (70%:30%); 35min, A:B (50%:50%); 35.1min, A:B (0%:100 %); 39.9 min, A:B (0%:100%) 40.0 min, A:B (90%:10%). Flow rate: 0.25mL / min; column temperature 30°C.
[0034] 2. Mass spectrometry conditions: Quantitative analysis was carried out in multiple reaction monitoring...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com