Perovskite catalyst used for autothermal reforming of ethanol for producing hydrogen and preparation method thereof
An autothermal reforming and catalyst technology, which is applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., can solve problems such as catalyst deactivation and reduce selectivity. , The effect of inhibiting the dehydration activity of alcohols and improving the reducibility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
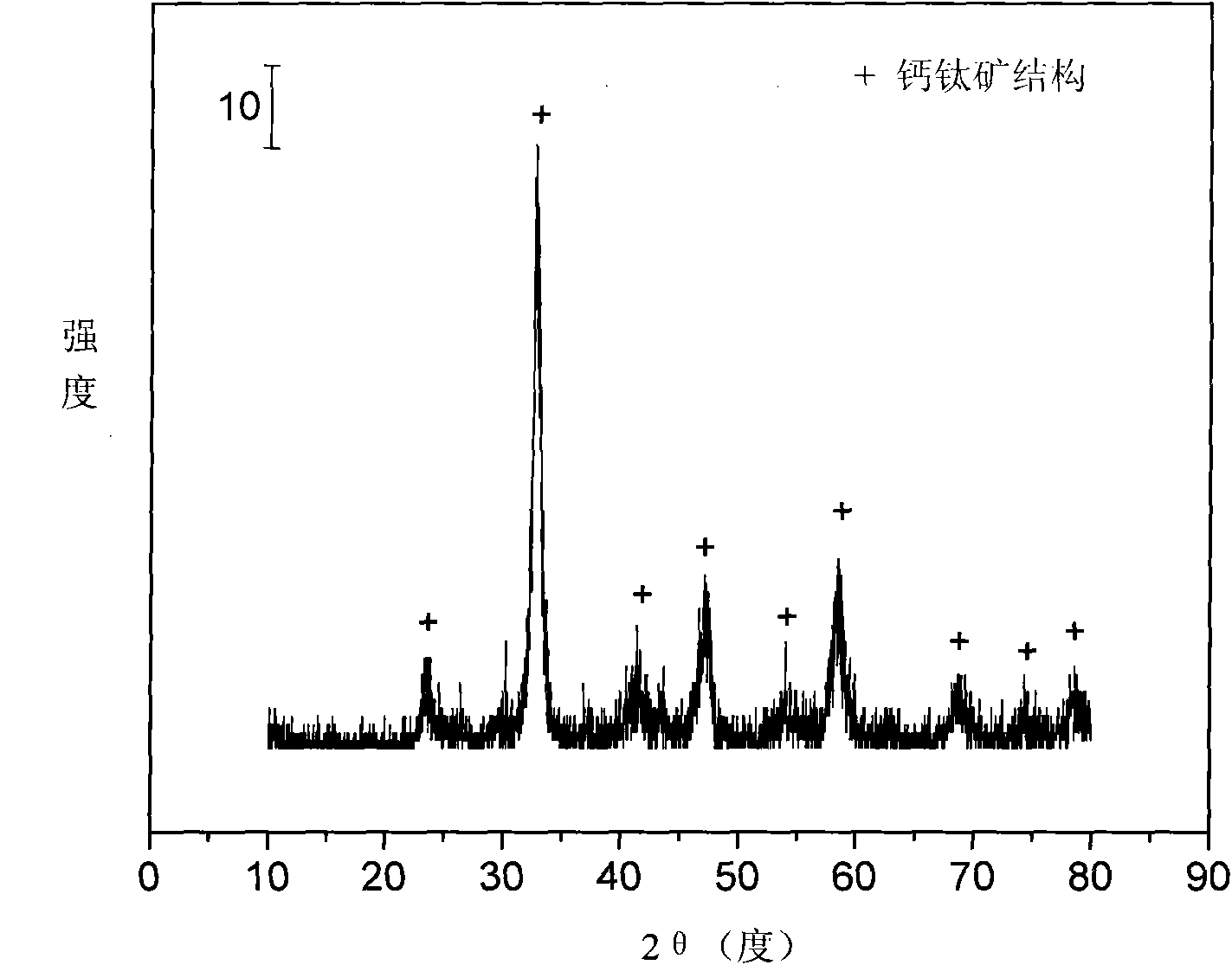
[0027] Weigh 10.669 grams of Ni (NO 3 ) 2 ·6H 2 O, 17.651 grams of La (NO 3 ) 2 ·6H 2 O and 1.649 g of Fe(NO 3 ) 3 9H 2 O, add 82ml of deionized water to make solution #1. Weigh 15.607 g of NH 2 CH 2 COOH was added to 104ml of deionized water to make solution #2. Subsequent steps were the same as those in Reference Example 1 to obtain catalyst CUT-PRV-LNF-101. The weight composition of the catalyst is as follows: the nickel oxide content is 28.2%, the dilanthanum trioxide content is 68.4%, and the ferric oxide content is 3.4%.
[0028] The activity of the catalyst is evaluated in the autothermal reforming reaction of ethanol. The reaction conditions are temperature 550°C, water / ethanol / oxygen molar ratio 3 / 1 / 0.5, normal pressure, space velocity 11000h -1 , the ethanol conversion rate is stable at 100%, while by-products such as methane and ethane are suppressed, and the hydrogen production rate is stable at about 3.2molH 2 / molEtOH, no deactivation phenomenon such...
Embodiment 2
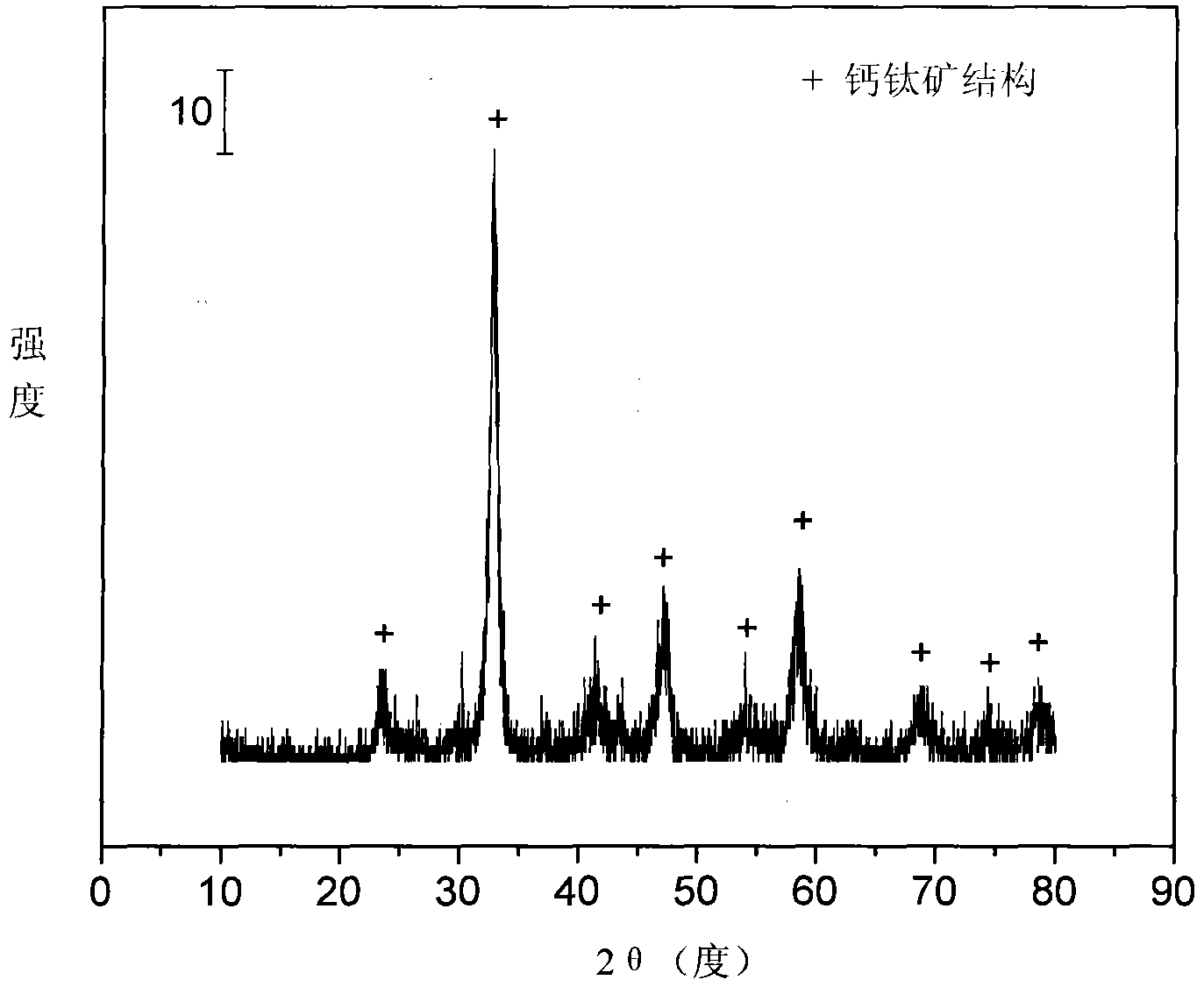
[0030] Weigh 11.134 grams of Ni (NO 3 ) 2 ·6H 2 O, 14.738 grams of La (NO 3 ) 2 ·6H 2 O, 1.719 grams of Fe(NO 3 ) 3 9H 2 O and 1.801 g of Sr(NO 3 ) 2 , was added to 85ml of deionized water to make solution #1. Weigh 14.372 g of NH 2 CH 2 COOH was added to 104ml of deionized water to make solution #2. Subsequent steps were the same as those in Reference Example 1 to obtain catalyst CUT-PRV-LSNF-101. The weight composition of the catalyst is as follows: the content of nickel oxide is 29.7%, the content of dilanthanum trioxide is 57.6%, the content of ferric oxide is 3.5%, and the content of strontium oxide is 9.2%.
[0031] The activity of the catalyst is evaluated in the autothermal reforming reaction of ethanol. The reaction conditions are temperature 550°C, water / ethanol / oxygen molar ratio 3 / 1 / 0.5, normal pressure, space velocity 11000h -1 , the ethanol conversion rate is stable at 100%, while by-products such as methane and ethane are suppressed, and the hydrog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| heating value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com