Olivine nickel-based catalyst for preparing hydrogen through autothermal reforming of acetic acid
An autothermal reforming, olivine-type technology, used in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, hydrogen, etc., can solve problems such as catalyst deactivation, achieve reduced selectivity, The effect of increasing the number of catalytic active centers and increasing the specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Weigh 23.106 grams of Ni (NO 3 ) 2 ·6H 2 O and 115.451 g of Mg(NO 3 ) 2 ·6H 2 O, was added to 530 mL of deionized water to make solution #1. Weigh 75.274 g of NaSiO 3 .9H 2 O, add 270 mL of deionized water to make solution #2. Weigh 10.0 g of NaOH and add it to 130 ml of deionized water to prepare solution #3. Solution #1 and solution #2 were subjected to co-precipitation reaction at 75 degrees Celsius, and the pH value of the reaction solution was controlled within the range of 10.5±0.5 by controlling the addition rate of solution #3. After the co-precipitation was completed, the resulting suspension Add 10.0 grams of polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer (P123, Mn=5800), stir for 2 hours, transfer to the reaction kettle, and place at 170 degrees Celsius In the oven, keep it for 36 hours, then filter, wash, and dry the suspended matter in the reactor, and then roast at 800 degrees Celsius to get the catalyst CUT-HS-N-101 ...
Embodiment 2
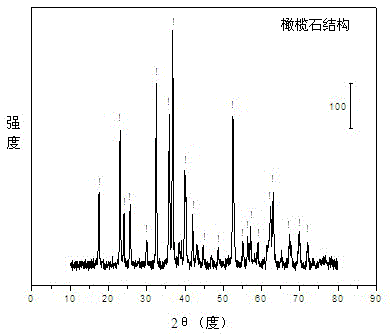
[0031] Weigh 22.565 grams of Ni (NO 3 ) 2 ·6H 2 O, 54.005 grams of Mg (NO 3 ) 2 ·6H 2 O and 62.696 g of Fe(NO 3 ) 3 .9H 2 O, was added to 530 mL of deionized water to make solution #1. Weigh 63.008 grams of NaSiO 3 .9H 2 O, add 230 mL of deionized water to make solution #2. Weigh 10.0 g of NaOH and add it to 130 ml of deionized water to prepare solution #3. Co-precipitate reaction of solution #1 and solution #2 at 75 °C, and control the pH value of the reaction solution within the range of 10.5±0.5 by controlling the addition rate of solution #3. After the co-precipitation is completed, the resulting suspension Add 10.0 g of polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer (P123, Mn=5800), stir for 2 hours, and transfer to the reaction kettle. Subsequent steps are the same as in Example 1 to obtain the catalyst CUT-HS-NF-201, whose typical olivine structure can be obtained from the attached figure 1 At the same time, the scanning electr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com