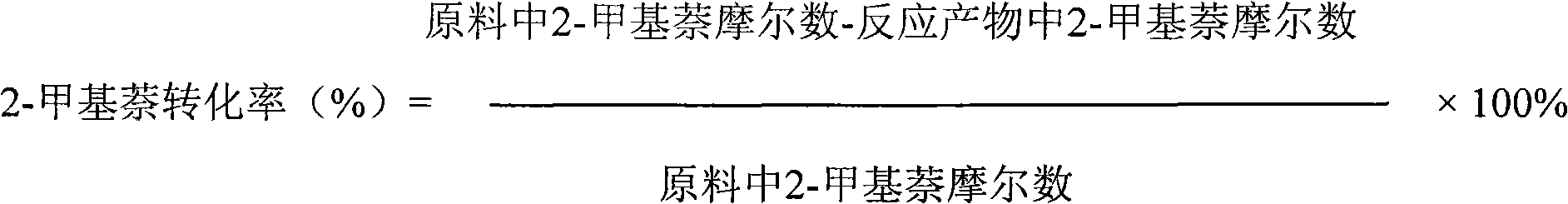
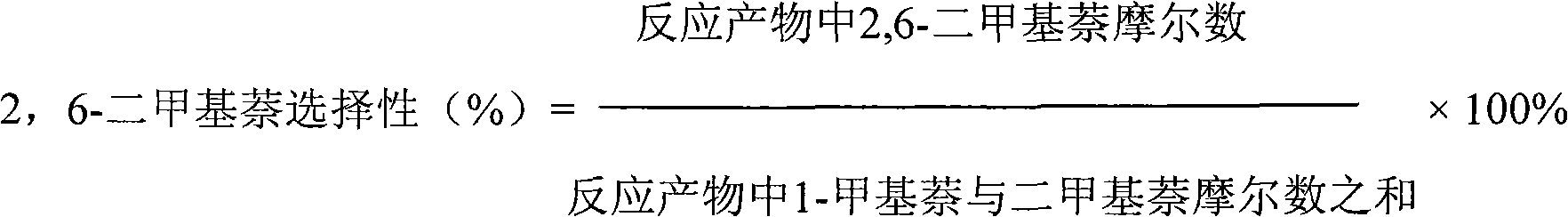Catalyst used for alkylation of methanol, C10 aromatic hydrocarbons and 2-methylnaphthalene for synthesizing 2,6-dimethylnaphthalene
A kind of methyl decalin, carbon ten aromatic hydrocarbon technology, applied in physical/chemical process catalyst, hydrocarbon, hydrocarbon and other directions, can solve the process of limiting industrial application, low methyl naphthalene conversion rate, volatile catalyst It can achieve high reactivity and product selectivity, improve selectivity and catalytic stability, and inhibit isomerization and other side reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Using SiO 2 / Al 2 o 3 42g of hydrogen-type ZSM-5 molecular sieve and 18g of Al with a molar ratio of 290 2 o 3 Mix, then add 3.6g of Tianqing powder and mix evenly, then add a certain amount of 3wt% dilute nitric acid as a binder, knead well, carry out extrusion molding, after drying at 85°C, bake at 540°C for 4 hours, using a temperature program , the heating rate was 3°C / min; the obtained HZSM-5 was designated as Catalyst Ⅰ.
Embodiment 2
[0025] The HZSM-5 molecular sieve prepared by the method described in Example 1 was impregnated with the obtained 10g molecular sieve in 9ml, 21.0wt% concentration of nickel nitrate aqueous solution, then dried at 85°C for 24 hours, and then calcined at 540°C for 4 Hours, using a temperature program with a heating rate of 3°C / min; 6.0wt% NiO / HZSM-5 was obtained, which was designated as Catalyst II.
Embodiment 3
[0027] Using the 6.0wt% Ni / HZSM-5 prepared by the method described in Example 2, after impregnating the prepared 10g molecular sieve in 9ml, 49.7wt% concentration of magnesium nitrate aqueous solution, dry at 85°C for 24 hours, and then dry at 540°C Calcined at lower temperature for 4 hours, using a temperature program with a heating rate of 3°C / min; 14.0wt% MgO / 6.0wt% NiO / HZSM-5 was obtained, which was designated as Catalyst III.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com