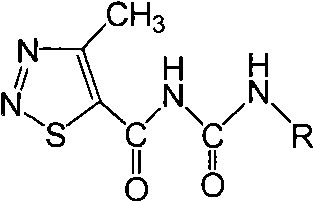
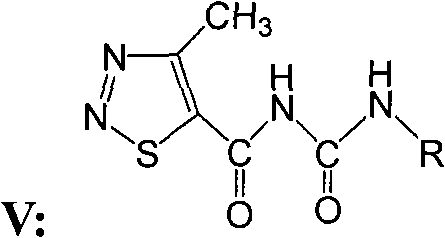
4-methyl-1,2,3-thiadiazole-5-formyl urea compounds and preparation method and application thereof
A kind of technology of thiadiazole and compound, applied in the field of 4-methyl-1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
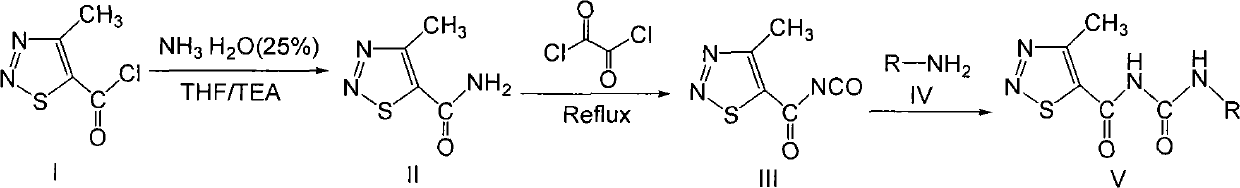
[0035] Preparation of intermediate 4-methyl-1,2,3-thiadiazole-5-carboxamide Ⅱ
[0036] In a 500 ml two-necked flask, add 30 ml of 25% ammonia water, 5 ml of triethylamine, and 50 ml of tetrahydrofuran, and drop 5 grams of 4- Methyl-1,2,3-thiadiazole-5-carbonyl chloride I was added dropwise in 15 minutes. Stirring was continued for 2 hours under ice-cooling and then at room temperature for 7 hours. After the reaction was completed, separate the liquid with a separatory funnel, extract the aqueous layer 3 times with 15 ml of tetrahydrofuran, combine the organic layers, dry with anhydrous sodium sulfate overnight, remove the anhydrous sodium sulfate by suction filtration, remove the solvent by rotary evaporation, and there is a white flaky solid Generated as 4-methyl-1,2,3-thiadiazole-5-carboxamide II.
Embodiment 2
[0038] Preparation of intermediate 4-methyl-1,2,3-thiadiazole-5-formyl isocyanate Ⅲ
[0039] Take a 100 ml three-necked flask, add 2 grams of 4-methyl-1,2,3-thiadiazole-5-carboxamide II and 15 ml of 1,2-dichloroethane, stir to make the amide in the solvent Disperse evenly, add 4.3 g of oxalyl chloride diluted with 5 ml of 1,2-dichloroethane dropwise with a dropping funnel under ice bath, dropwise in 30 minutes, then stir at room temperature for 1 hour, and then heat to 80 degrees Celsius After reflux for 7 hours, the reaction solution changed from white to turbid to a light yellow transparent solution, and the color gradually became darker after heating. After the reaction was completed, the solvent was removed by rotary evaporation to obtain the product 4-methyl-1,2,3-thiadiazole-5-formylisocyanate III, which was directly used in subsequent reactions without further purification.
Embodiment 3
[0041] Compound GDD-1: Synthesis and structure identification of N-(4-nitrophenyl)-N’-(4-methyl-1,2,3-thiadiazole-5-formyl)urea
[0042] Add 2.7 millimoles of p-nitroaniline and 20 milliliters of 1,2-dichloroethane into a 100-ml round-bottomed flask, stir until the p-nitroaniline is completely dissolved, then add 3 mmoles of 4-methyl-1 dropwise under stirring, 2,3-Thiadiazole-5-formyl isocyanate, after 15 minutes of dropwise addition, a precipitate was formed immediately, stirred at room temperature for 8 hours, after the reaction was completed, stood still, separated by suction filtration to obtain a solid product, and the filtrate was rotatably evaporated to remove the solvent , with volume ratio of 1:3 ethyl acetate: sherwood oil recrystallization and put it in the refrigerator, precipitated solid, combined solid product, with volume ratio of 1:3 ethyl acetate: sherwood oil washed and dried to obtain the product; Melting point: 178-180 degrees Celsius, yield 58%. NMR data ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com