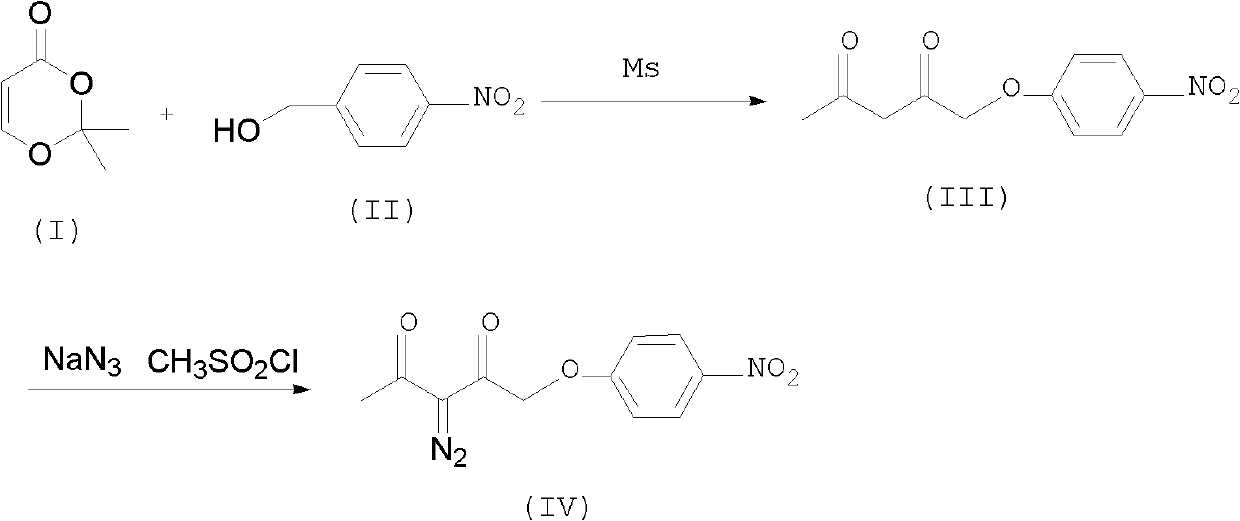
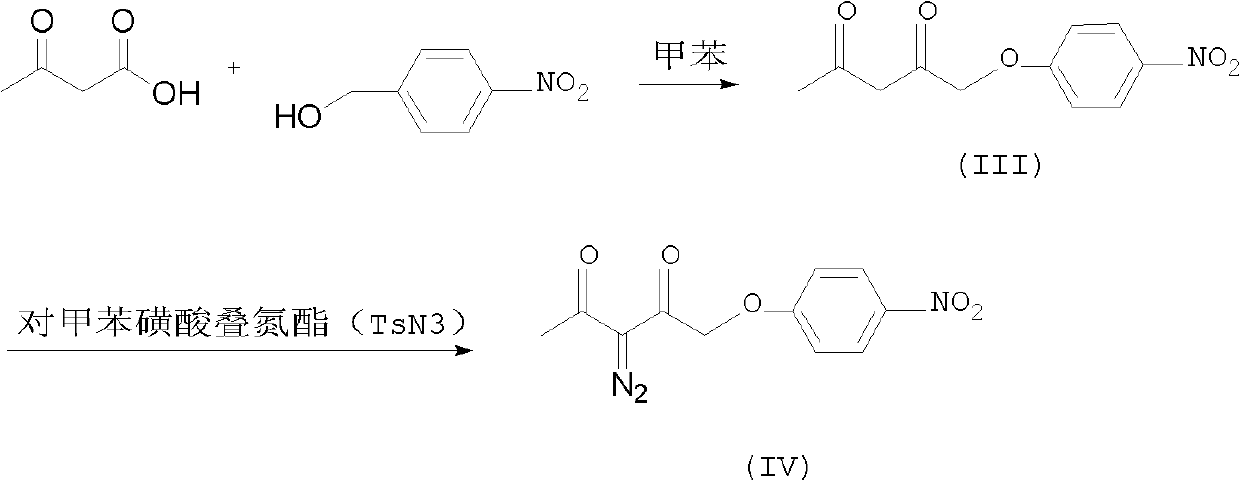
Preparation method of p-nitrobenzyl 2-diazoacetoacetate
A technology of diazoacetate and p-nitrobenzyl ester, applied in directions such as organic chemistry, can solve the problems of complex process route, difficult availability of diazonium reagents, expensive catalysts, etc., and achieves easy industrial production, high yield, simplification and the like. The effect of the process operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1, the preparation of p-nitrobenzyl acetoacetate i.e. compound (III):
[0019] Add 43.5g of p-nitrobenzyl alcohol and 150g of toluene to the flask, then add 45.9g of 2.2-dimethyl-1.3-oxcyclohex-4-en-6-one, then raise the temperature and stir the reaction at 30-35°C for 5.0h HPLC tracking reaction Finish, cool down 20~25 ℃ for later use.
Embodiment 2
[0020] Embodiment 2, the preparation of 2-diazoacetoacetic acid p-nitrobenzyl ester compound (VI):
[0021] In another flask, add 9.0 g of sodium bicarbonate, 23.0 g of sodium azide, 2.0 g of phase transfer catalyst tri-n-butyl methyl ammonium chloride, and 160 g of purified water, start stirring, raise the temperature to 20-30°C, and start adding formazan Sulfonyl chloride 38.0g, temperature control at 20-30°C for about 40 minutes to complete the dropwise addition, start dropwise adding the standby solution after the reaction in Example 1, temperature control at 25-30°C, dropwise for about 1.0h, at 25-30°C Keep warm for 4.0 hours, HPLC detects that the reaction is complete, and then lower the temperature by 0-5°C.
[0022] Insulate for 1.0h, filter, drain, dig out the filter cake, put into another flask, add 200g of water, 200g of toluene, stir to cool down to 0-5°C, continue stirring for 2.0h, filter, drain, dig out the filter cake, at 50-60 After drying under reduced press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com