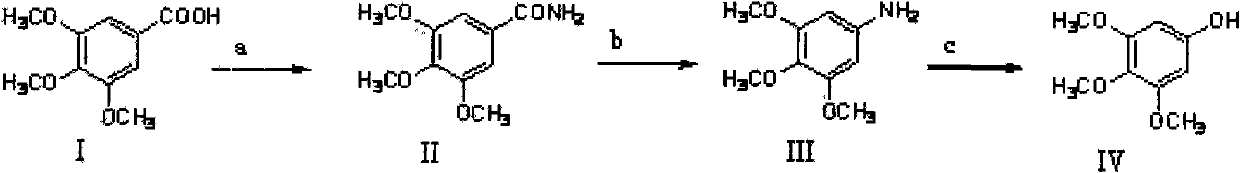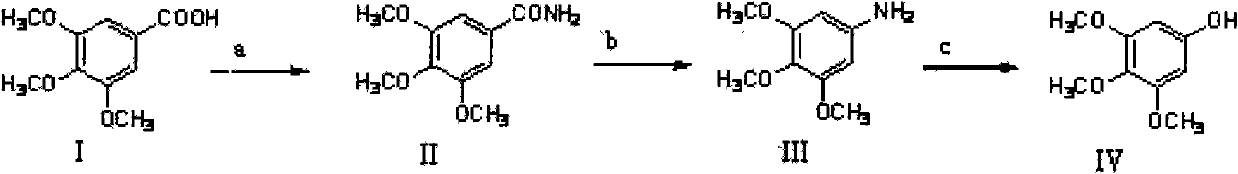Method for preparing 3,4,5-trimethoxyphenol
A technology for trimethoxyphenol and trimethoxybenzamide is applied in the field of preparation of pharmaceutical intermediates 3, can solve the problems of difficulty in obtaining, high price, low product yield and the like, and achieves simple and practical operation, safe use, high yield and the like. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Step 1: Synthesis of 3,4,5-trimethoxybenzamide
[0041] Weigh 106g (0.5mol) of 3,4,5-trimethoxybenzoic acid and place it in a 1000ml reaction flask, add 150ml of dichloromethane and 6 drops of DMF, add SOCl 2A total of about 45ml. Then stirred and heated to 30° C. for reflux reaction for 5 hours. Let cool, and try to remove all solvent and unreacted SOCl by distillation under reduced pressure 2 , add 200ml of acetone to dissolve the residual solid (i.e. acid chloride) and then slowly add dropwise to 500ml of 25% concentrated ammonia water at 0°C for about 30min while vigorously stirring. After dropping, continue to stir for 20 minutes, filter, collect the precipitate, wash with a small amount of cold water, press dry, and dry at 80°C to obtain a white powdery solid, namely 3,4,5-trimethoxybenzamide. Melting point: 172-175°C, weighing: 98.5g, yield: 93%. 1 HNMR (CDCl 3 ): δ7.06(s, 2H), δ3.92(s, 6H), δ3.91(s, 3H).
[0042] Step 2: Synthesis of 3,4,5-trimethoxyanilin...
Embodiment 2
[0049] Step 1: Synthesis of 3,4,5-trimethoxybenzamide
[0050] Weigh 159g (0.75mol) of 3,4,5-trimethoxybenzoic acid into a 1000ml reaction bottle, add 150ml of dichloromethane and 6ml of pyridine, and add phosphorus oxychloride to a total of about 45ml. Then stirred and heated to 80° C. for reflux reaction for 5 hours. Let cool, remove all solvents and unreacted phosphorus oxychloride by distillation under reduced pressure as much as possible, add 200ml acetone to dissolve the residual solid (i.e. acid chloride), then slowly add dropwise to 500ml 20% concentrated ammonia water at 10°C, drop it in about 30 minutes, and vigorously Stir. After dropping, continue to stir for 20 minutes, filter, collect the precipitate, wash with a small amount of cold water, press dry, and dry at 80°C to obtain a white powdery solid, namely 3,4,5-trimethoxybenzamide. Melting point: 172-175°C, weighing: 143.1 g, yield: 90%. 1 HNMR (CDCl 3 ): δ7.06(s, 2H), δ3.92(s, 6H), δ3.91(s, 3H).
[0051] S...
Embodiment 3
[0058] Step 1: Synthesis of 3,4,5-trimethoxybenzamide
[0059] Weigh 106g (0.5mol) of 3,4,5-trimethoxybenzoic acid into a 1000ml reaction bottle, add 150ml of dichloromethane and 6 drops of DMF, and add phosphorus pentachloride to a total of about 45ml. Then stirred and heated to 50° C. for reflux reaction for 5 hours. Let cool, remove all solvents and unreacted phosphorus pentachloride as much as possible by distillation under reduced pressure, add 200ml acetone to dissolve the residual solid (acyl chloride) and slowly add 500ml 30% concentrated ammonia water at 5°C dropwise for about 30 minutes. Stir. After dropping, continue to stir for 20 minutes, filter, collect the precipitate, wash with a small amount of cold water, press dry, and dry at 80°C to obtain a white powdery solid, namely 3,4,5-trimethoxybenzamide. Melting point: 172-175°C, weighing: 100.7g, yield: 95%. 1 HNMR (CDCl 3 ): δ7.06(s, 2H), δ3.92(s, 6H), δ3.91(s, 3H).
[0060] Step 2: Synthesis of 3,4,5-trimeth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com