Anti-tumor controlled release nanocomposite and preparation method thereof
A nano-composite, anti-tumor drug technology, applied in the field of anti-tumor controlled-release nano-composite, can solve the problems of unfavorable biological application, poor water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
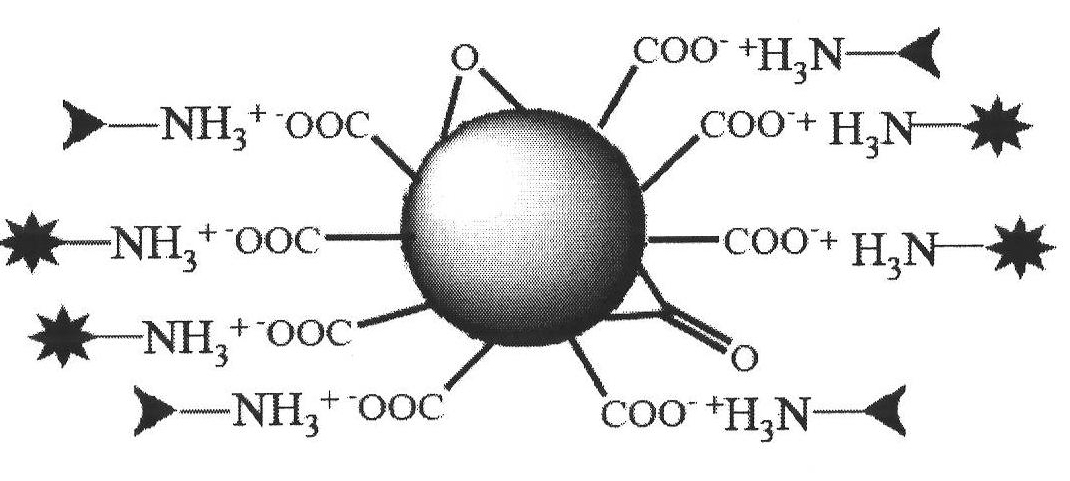
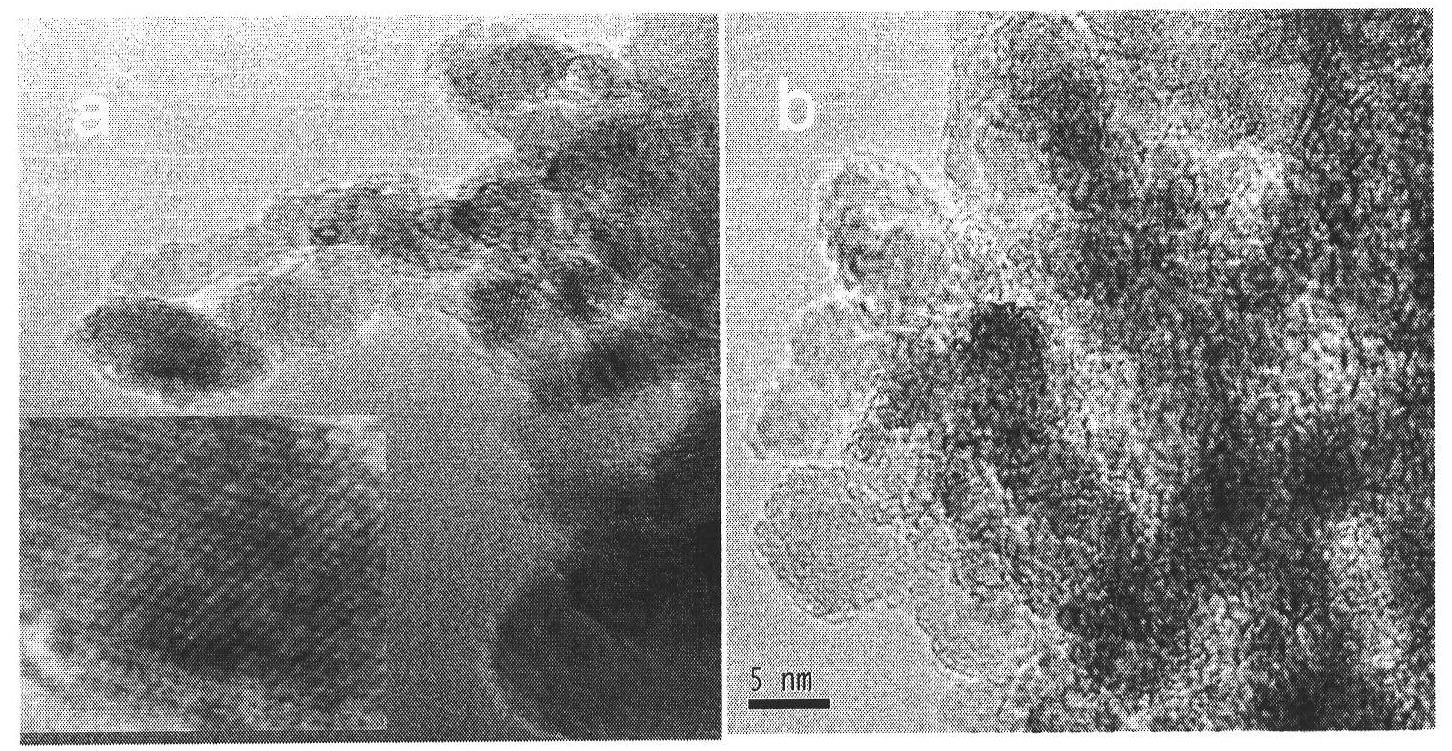
[0043] The main steps of the preparation of the nano-composites for the controlled release of anti-tumor drugs provided by the present invention are as follows: the nano-diamonds are subjected to strong acid carboxylation treatment, and ultrasonically dispersed in 50% DMSO / H 2 O, add doxorubicin (also paclitaxel or methotrexate, etc.) connected with the enzyme self-degrading chain, and stir magnetically at room temperature. The obtained complex was dispersed in triple-distilled water, the membrane-penetrating peptide TAT was added, magnetically stirred at room temperature, and dried to obtain a doxorubicin controlled-release nanocomplex (TAT-ND-Phe-Lys-PABC-DOX).
[0044] In the method for preparing the above-mentioned doxorubicin controlled-release nanocomposite of the present invention, the treatment process of nano-diamonds through strong acid carboxylation is: adding nano-diamonds to H 2 SO 4 -HClO 4 (3:1) strong acid mixed solution, stirred at 40°C for 24h, refluxed in ...
Embodiment 1
[0052] 1) Preparation of carboxylated nanodiamonds:
[0053] Add 0.5 g nanodiamonds to 20 mL H 2 SO 4 -HClO 4 (3:1) in the mixed solution, stirred at 40°C for 24h, centrifuged, refluxed in 0.1M NaOH aqueous solution at 90°C for 2h, centrifuged, then refluxed in 0.1M HCl aqueous solution at 90°C for 2h, centrifuged, washed with three distilled water several times, Vacuum dry.
[0054] 2) Preparation of ND-Phe-Lys-PABC-DOX nanocomposite:
[0055] Add 4 mg of carboxylated nanodiamonds to 50% dimethyl sulfoxide aqueous solution, and disperse them by ultrasonication at room temperature for 4 h. Add 0.4 mg of Phe-Lys-PABC-DOX to the dispersed nano-diamond solution, stir magnetically at room temperature for 24 hours to completely bind Phe-Lys-PABC-DOX to the nano-diamond, centrifuge, discard the supernatant, and wash with three-distilled water.
[0056] 3) Preparation of TAT-ND-Phe-Lys-PABC-DOX nanocomposite:
[0057] After ultrasonically dispersing the ND-Phe-Lys-PABC-DOX nano...
Embodiment 2
[0065] Study on the adsorption isotherm of nanodiamonds and Phe-Lys-PABC-DOX:
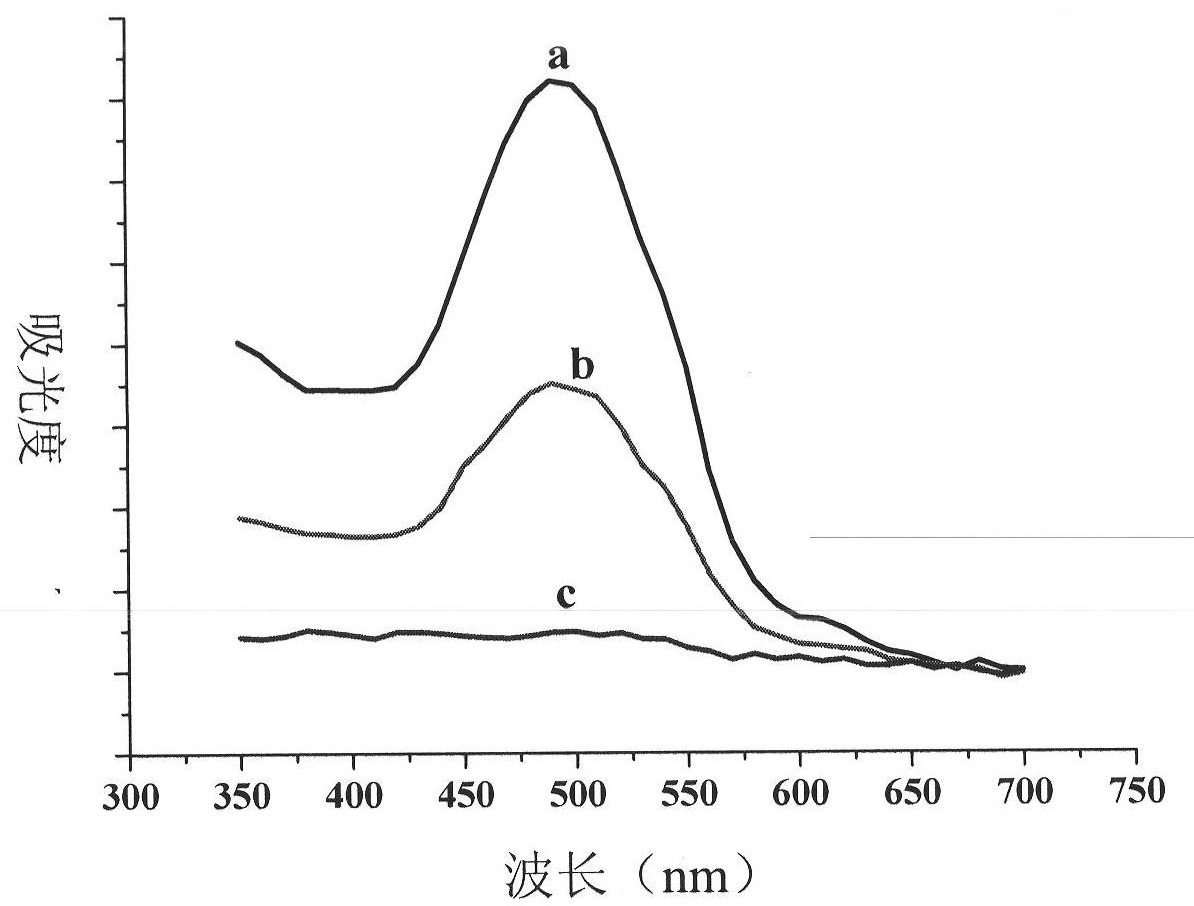
[0066] Add 1 mg of nanodiamonds to the centrifuge tubes respectively, and add Phe-Lys-PABC-DOX with a mass concentration of 0.08, 0.11, 0.14, 0.17, 0.20, and 0.23 mg / mL to the centrifuge tubes in sequence. Turn, shake for 28 hours, centrifuge, take the supernatant in a cuvette, measure the ultraviolet absorption of Phe-Lys-PABC-DOX at 492nm, calculate the supernatant amount according to its standard curve, and further calculate the adsorption amount. According to the formula
[0067] Langmuir isotherm equation: To Ce vs. Do linear equation: y=1.3323x+0.3279, R 2 = 0.3666. According to the formula Freundlich isotherm equation in LogC e to Logq e Do the linear equation: y=0.8654x+0.2243R 2 = 0.9112.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com