Methods and compositions using KLOTHO-FGF fusion polypeptides
A technology of fusion polypeptide and composition, applied in the field of treating testicular pathology, fusion polypeptide, Klotho fusion polypeptide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
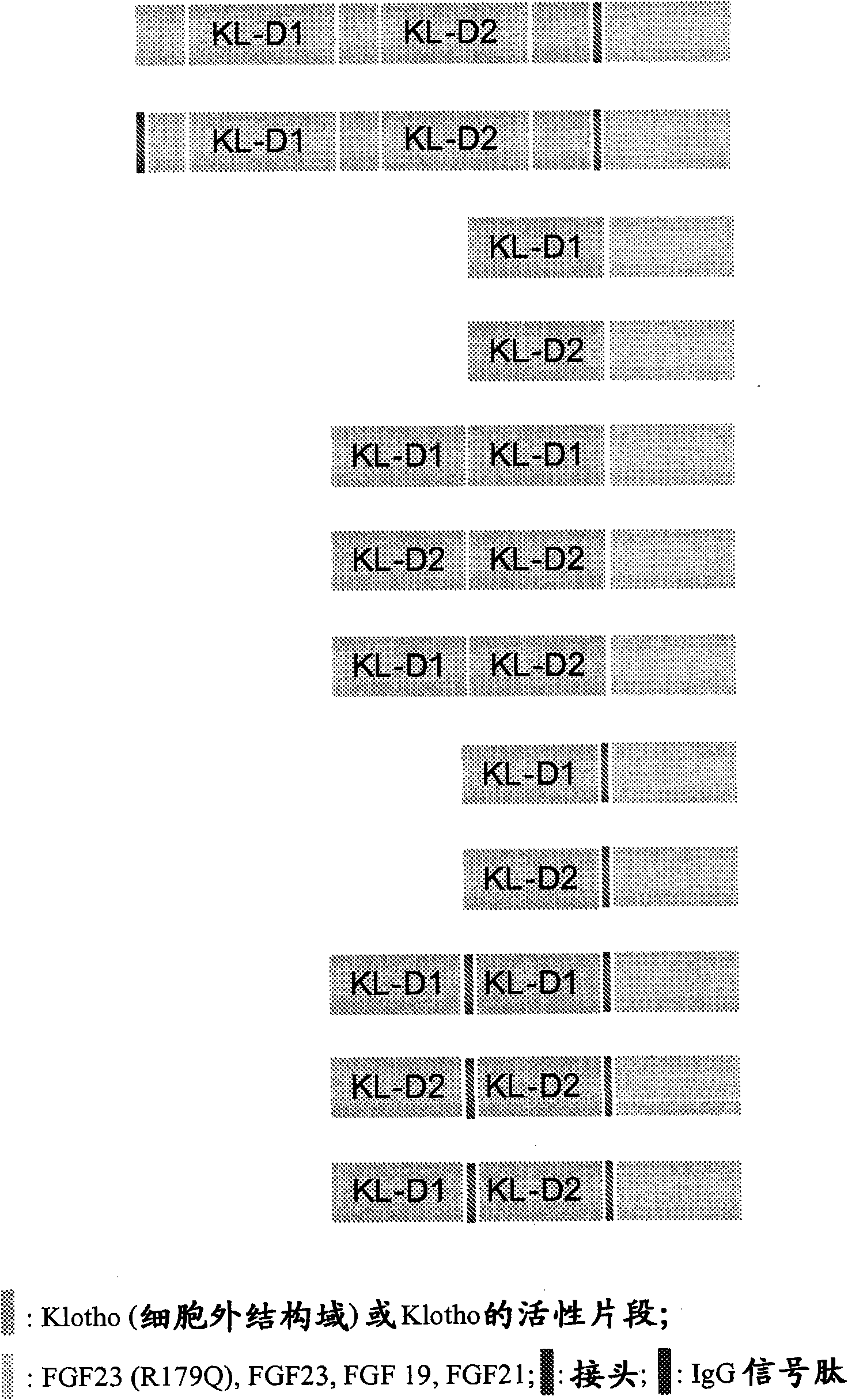
[0208] Expression and purification of embodiment 1.Klotho fusion polypeptide
[0209] Expression of Klotho fusion polypeptide
[0210] The polypeptide of the present invention is prepared by transiently transfecting HEK293T cells with an expression vector encoding a Klotho fusion polypeptide having the extracellular domain of αKlotho and the FGF23(R179Q) variant. Conditioned media containing the expressed polypeptides were generated by transient transfection of the corresponding expression plasmids for Klotho, FGF23 and Klotho-FGF23(R179Q) fusion proteins. Transfections were performed in 6-well plates using Lipofectamine 2000 (Invitrogen, catalog #11668-019). Five hours after transfection, the transfection mixture was replaced with 3 ml DMEM plus 1% FBS. Conditioned medium was collected 72 hours after the addition of 3 ml DMEM plus 1% FBS. Conditioned medium samples from various transiently transfected HEK293T cells were separated by SDS-polyacrylamide gel electrophoresis (...
Embodiment 2
[0214] Example 2. In vitro assays to assess the activity of Klotho fusion polypeptides
[0215] Egr-1-luciferase
[0216] The expressed αKlotho fusion polypeptides were tested for biological activity in an Egr-1-luciferase reporter assay. Binding of the Klotho fusion polypeptide to the FGF23 receptor results in downstream activation of Egr-1 and expression of a luciferase reporter regulated by the Egr-1 promoter. The Egr-1-luciferase reporter gene was constructed based on the report of Urakawa et al. (Nature, 2006, Vol. 444, 770-774). HEK293T cells seeded in 48-well poly-D-lysine plates were transfected with the Egr-1-luciferase reporter gene together with the transfection labeling reporter gene (renilla luciferase). Five hours after Egr-1 luciferase reporter transfection, the transfection mixture was replaced with 3 ml DMEM plus 1% FBS. Conditioned medium was collected 72 hours after the addition of 3 ml DMEM plus 1% FBS. After 5 hours, the transfection mixture was replac...
Embodiment 3
[0222]Example 3. In vitro assays to assess the effects of Klotho fusion polypeptides on muscle cells.
[0223] The biological effects of the expressed Klotho fusion polypeptides were tested on C2C12 myoblasts. Treatment of C2C12 myoblasts with IGF-1, FGF2 or sKlotho-FGF23 results in myotube growth and phosphorylation of signaling proteins. C2C12 myoblasts were seeded at a density of 40,000 cells / well on 6-well poly-D-lysine and fibronectin-coated plates in growth medium [(3 parts DMEM and 1 part F12), 10% FBS, 1 % Glut; 1% P / S; 1% Linoleic Acid; 0.1% ITS: [Insulin (10 mg / ml), Transferrin (5.5 mg / ml) and Selenium (5 ng / ml)]. After myoblasts reached confluence (3 days), the medium was changed to differentiation medium (DMED with 2% horse serum; 1% Glut; 1% P / S).
[0224] For myotube diameter experiments, 3 days after confluence, the medium was changed to differentiation medium in which IGF-1 (10 nM), FGF2 (20 ng / ml) or sKlotho-FGF23 (20nM) to treat the cells for 24 hours. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com