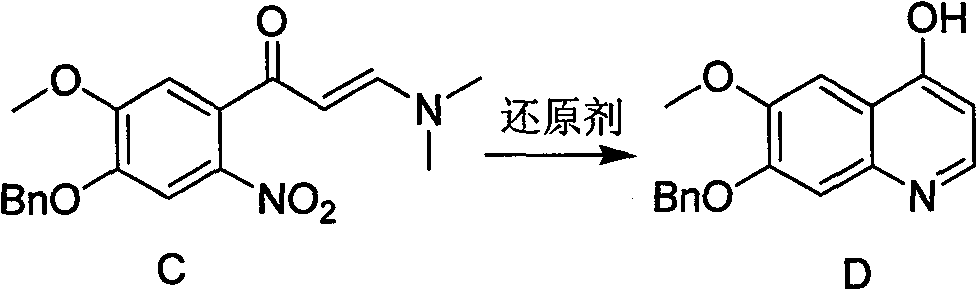
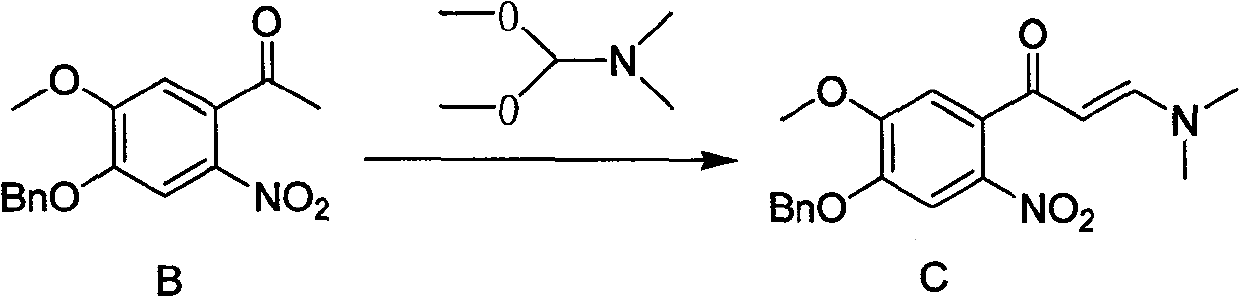
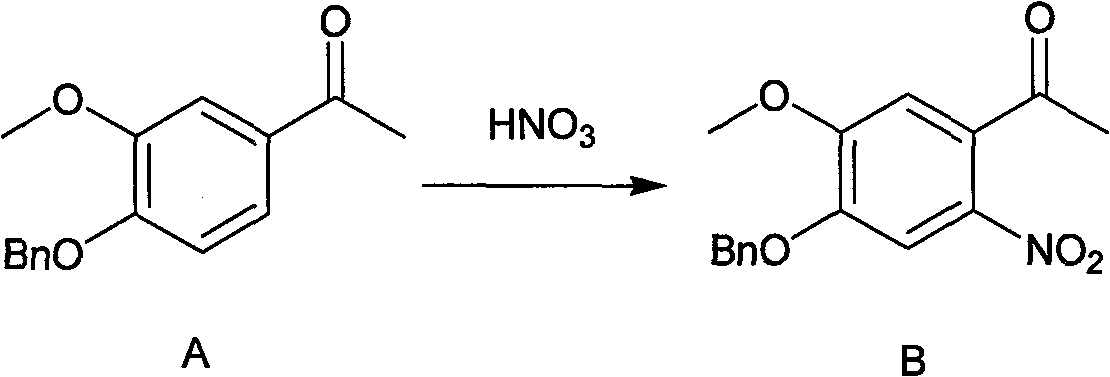
Synthesis method of 7- benzyloxy-6-methoxyl-4-hydroxyquinoline
A technology of hydroxyquinoline and synthetic method, applied in the direction of organic chemistry, etc., can solve problems such as unsuitable for industrial production, harsh reaction conditions, complicated post-processing, etc., and achieve the effect of avoiding column separation process, high conversion rate, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of 4-benzyloxy-5-methoxy-2-nitroacetophenone (compound B)
[0050] Compound A (67 g, 0.25 mol) was dissolved in dichloromethane (450 ml), cooled to -5°C with stirring. After 65% nitric acid (24 g, 0.25 mol, 17 ml) was dissolved in acetic acid (250 ml), it was added dropwise to the acetic anhydride solution of compound A, stirred at 0-5° C. for 20 hours, and LCMS detected that the reaction was complete.
[0051] After concentrating under reduced pressure to remove dichloromethane, the reaction solution was poured into stirred ice water, the precipitated solid was filtered, washed with water and beaten with absolute ethanol, and the solid was filtered and dried to obtain compound B, 62.8 g, yield 81%, liquid chromatography The detection purity is 96%.
Embodiment 2
[0052]Example 2 Preparation of 4-benzyloxy-5-methoxy-2-nitroacetophenone (compound B)
[0053] Compound A (67 g, 0.25 mol) was dissolved in acetic anhydride (450 ml), cooled to -5°C with stirring. After 65% nitric acid (44.9 g, 0.46 mol, 32 ml) was dissolved in acetic acid (450 ml), it was added dropwise to the acetic anhydride solution of compound A, stirred at 0-5° C. for 18 hours, and the reaction was detected by LCMS to be complete.
[0054] The reaction solution was poured into stirred ice water, and the precipitated solid was filtered, washed with water, slurried with absolute ethanol, and the solid was filtered and dried to obtain Compound B, 64.4 g, with a yield of 83% and a purity of 98% by liquid chromatography.
Embodiment 3
[0055] Example 3 Preparation of 4-benzyloxy-5-methoxy-2-nitroacetophenone (compound B)
[0056] Compound A (67 g, 0.25 mol) was dissolved in acetic anhydride (450 ml), cooled to -5°C with stirring. After 65% nitric acid (44.9 g, 0.46 mol, 32 ml) was dissolved in 96% sulfuric acid (240 ml), it was added dropwise in the acetic anhydride solution of compound A, stirred at 0-5° C. for 18 hours, and LCMS detected that the reaction was complete.
[0057] The reaction solution was poured into stirred ice water, the precipitated solid was filtered, washed with water, beaten with anhydrous 95% ethanol, and the solid was filtered and dried to obtain compound B, 66.0 g, yield 85%, purity 96% by liquid chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com