Externally applied pharmaceutical composition for treating skin fungal infection
A technology of topical medicine and skin fungus, which is applied in the field of medicine, can solve problems affecting the quality of life of patients, and achieve the effects of relieving skin burning sensation, shortening the anti-itching time, and enhancing antifungal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
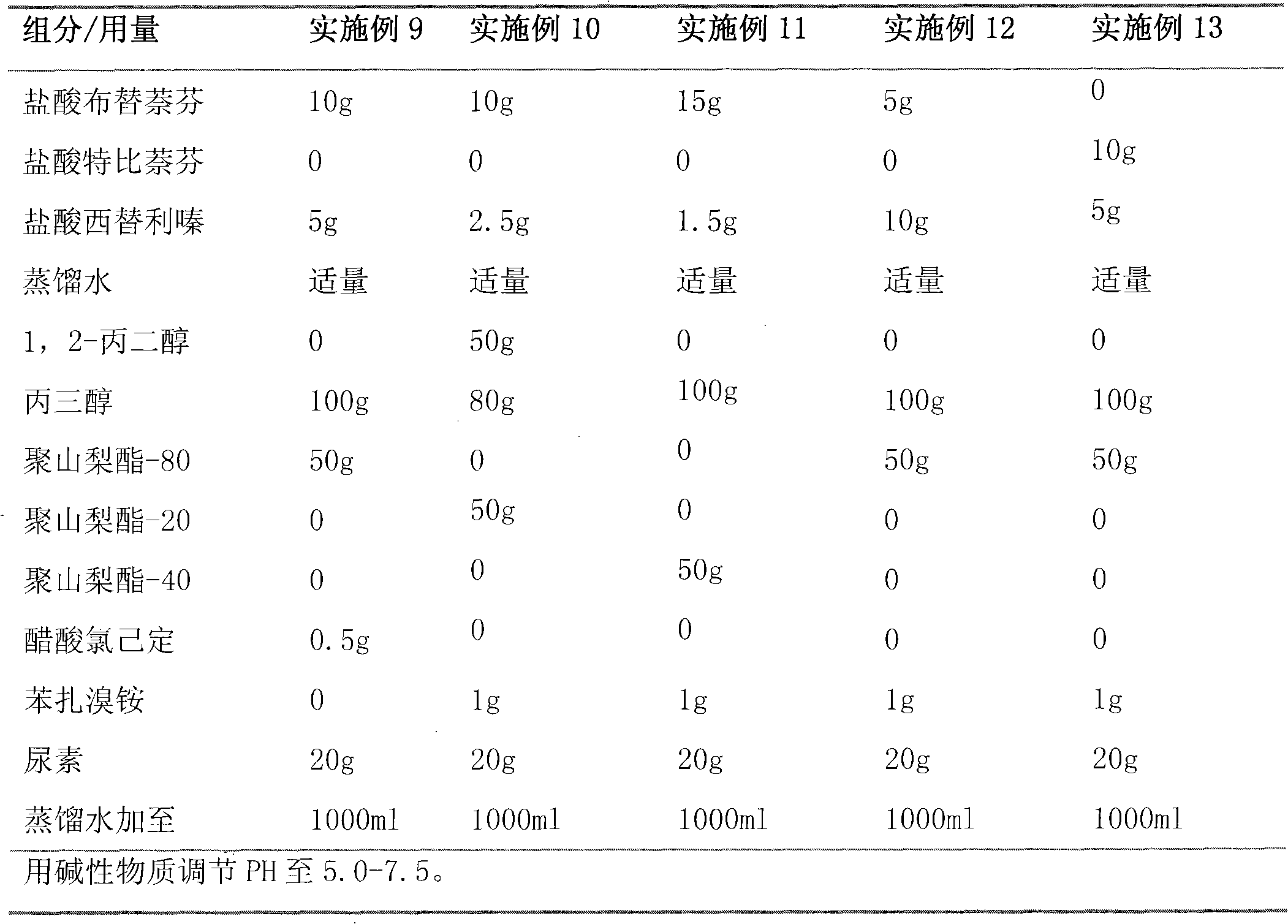
[0055] Embodiment 1 gel
[0056] Butenafine Hydrochloride 10g
[0057] Miconazole Nitrate 2.5g
[0058] Loratadine 2.5g
[0059] 1,2-propanediol 100g
[0060] Glycerol 50g
[0061] Carbopol 940 10g
[0062] Polysorbate-80 2g
[0064] Ethylparaben 1g
[0065] Distilled water up to 1000g
[0066] Preparation process: Dissolve butenafine hydrochloride, miconazole nitrate and loratadine in 1,2-propanediol, then add appropriate amount of distilled water and glycerin, then mix carbopol 940 with polysorbate-80 and 300ml of distilled water, mix well, dissolve sodium hydroxide in 100ml of distilled water, add supernatant and mix well, then dissolve ethylparaben in ethanol, add gradually and stir well, to obtain transparent gel.
Embodiment 2
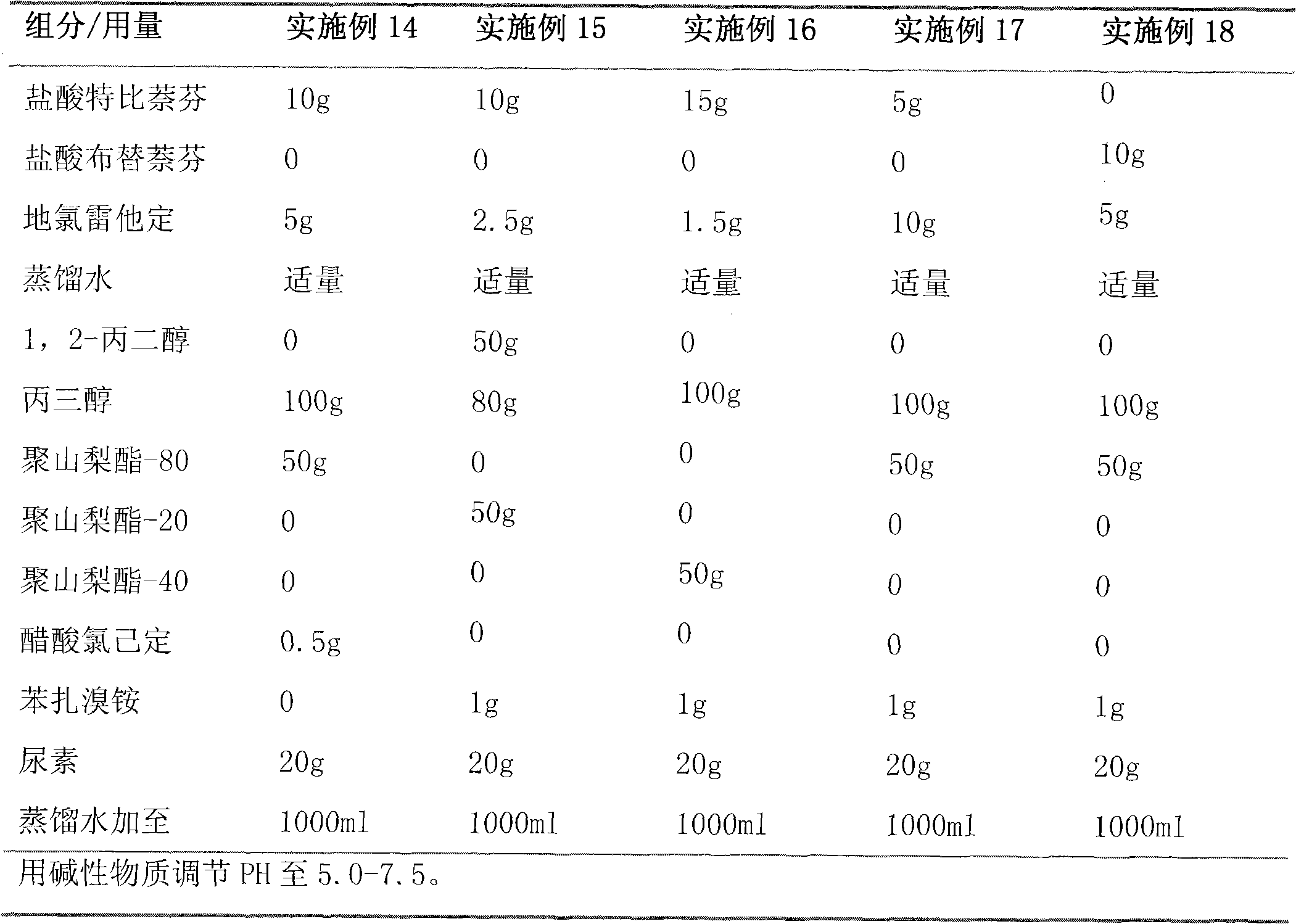
[0067] Embodiment 2 gel
[0068] Butenafine Hydrochloride 10g
[0069] Cetirizine Hydrochloride 5g
[0070] 1,2-propanediol 80g
[0071] Glycerol 100g
[0072] Carbopol 940 8g
[0073] Polysorbate 80 2g
[0074] Ethylparaben 1g
[0075] Distilled water up to 1000g
[0076] Triethanolamine to adjust pH to 6.0
[0077] Preparation process: Dissolve butenafine hydrochloride and cetirizine hydrochloride in 1,2-propanediol, then add appropriate amount of distilled water and glycerol, then mix carbopol 940 with polysorbate-80 and 300ml distilled water Mix evenly, adjust the pH value to 6.0 with triethanolamine, then dissolve ethylparaben in ethanol, add gradually and stir evenly, add distilled water to 1000g, and obtain a transparent gel.
Embodiment 3
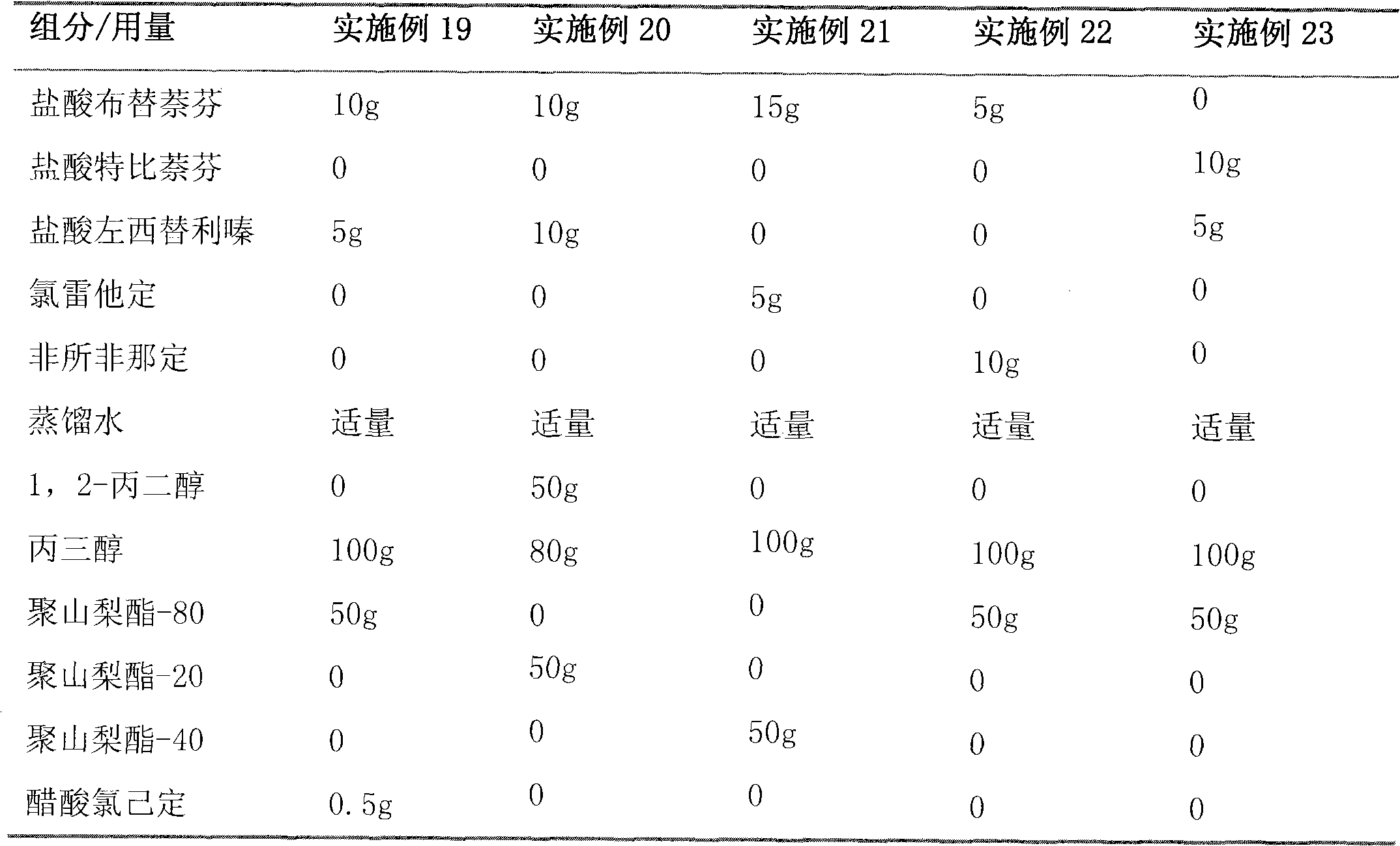
[0078] Embodiment 3 gel
[0079] Terbinafine Hydrochloride 10g
[0080] Cetirizine Hydrochloride 10g
[0081] 1,2-propanediol 100g
[0082] Glycerol 50g
[0083] Carbopol 940 10g
[0084] Polysorbate 80 2g
[0085] Ethylparaben 1g
[0086] Distilled water up to 1000g
[0087] Adjust pH to 7.0 with triethanolamine
[0088] The preparation process is the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com