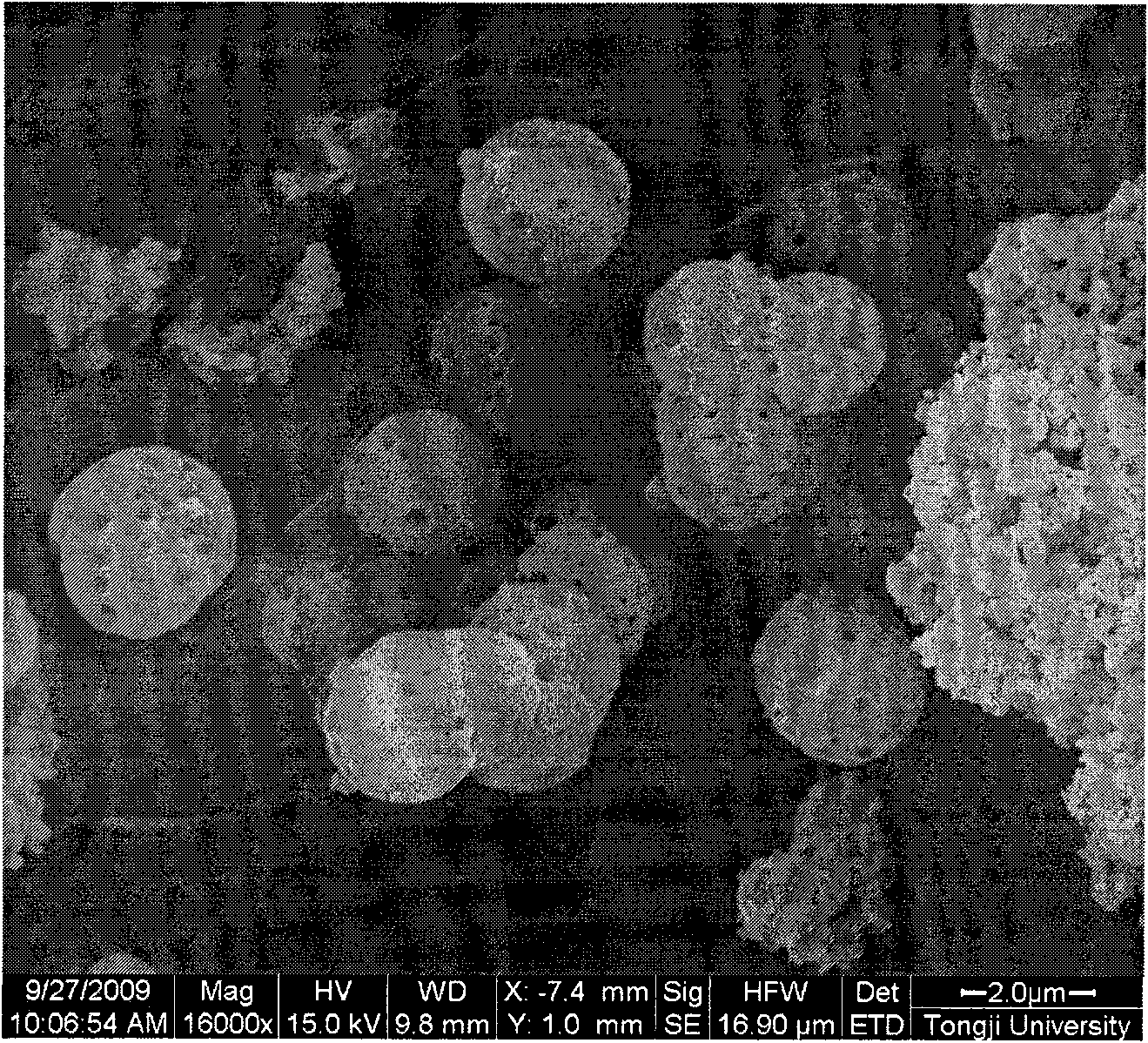Method for preparing nanocapsule powder of urea-formaldehyde-resin-encapsulated phase-change material
A technology of phase change materials and urea-formaldehyde resins, which is applied in the field of nanocapsule powder preparation, can solve problems such as environmental corrosion, phase instability, and limitations of phase change materials, and achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of prepolymer: first take by weighing 24.892g deionized water, 3g urea, all dissolve urea by stirring, then add 8.108g formaldehyde solution of 37% concentration by weight in the above solution, stir After uniformity, use a 10% triethanolamine aqueous solution to adjust its pH value to 8.5, then slowly drain the resulting mixture into a three-necked flask reaction device equipped with a thermometer, a condenser tube, and a constant pressure burette, and then place the The temperature of the water bath was raised to 70°C, and the reaction was carried out for 1 hour to obtain a prepolymer.
[0031] (2) Preparation of miniemulsion: Weigh 0.4g sodium dodecyl sulfate (SDS) and 0.12g hexadecane (CA), dissolve it in 30g deionized water, and add to it after the surfactant is fully dissolved After adding 6g of paraffin, the mixture was placed in an environment of 50°C to fully melt the paraffin, and then the mixture was immediately placed in an ultrasonic cell pu...
Embodiment 2
[0036] (1) Preparation of prepolymer: first take by weighing 26.595g deionized water, 2g urea, all dissolve urea by stirring, then add 5.405g formaldehyde solution of 37% concentration by weight in the above solution, stir After uniformity, use a 10% triethanolamine aqueous solution to adjust its pH value to 8.5, then slowly drain the resulting mixture into a three-necked flask reaction device equipped with a thermometer, a condenser tube, and a constant pressure burette, and then place the The temperature of the water bath was raised to 70°C, and the reaction was carried out for 1 hour to obtain a prepolymer.
[0037] (2) Preparation of miniemulsion: Weigh 0.4g sodium dodecyl sulfate (SDS) and 0.12g hexadecane (CA), dissolve it in 30g deionized water, and add to it after the surfactant is fully dissolved After adding 8g of paraffin, the mixture was placed in an environment of 50°C to fully melt the paraffin, and then the mixture was immediately placed in an ultrasonic cell pu...
Embodiment 3
[0041] (1) Preparation of prepolymer: first take by weighing 25.914g deionized water, 2.4g urea, all dissolve urea by stirring, then add 6.486g formaldehyde solution of 37% concentration by weight in the above solution, After stirring evenly, adjust its pH value to 8.5 with a 10% triethanolamine aqueous solution by weight, then slowly drain the resulting mixture into a three-necked flask reaction device equipped with a thermometer, a condenser tube, and a constant pressure burette, and then Raise the temperature of the water bath to 70°C and react for 1 hour to obtain a prepolymer.
[0042] (2) Preparation of miniemulsion: Weigh 0.4g sodium dodecyl sulfate (SDS) and 0.12g hexadecane (CA), dissolve it in 30g deionized water, and add to it after the surfactant is fully dissolved After adding 7.2g of paraffin, the mixture was placed in an environment of 50°C to fully melt the paraffin, and then the mixture was immediately placed in an ultrasonic cell pulverizer for ultrasonic tre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com