Method for preparing fluoroethylene carbonate
A technology of fluoroethylene carbonate and chloroethylene carbonate is applied in the field of preparation of fluoroethylene carbonate, and can solve the problems of side reaction tar and fluoroethylene carbonate being difficult to meet the requirements for use, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Preparation method of fluoroethylene carbonate
[0043] The invention provides a kind of preparation method of fluoroethylene carbonate, the method comprises the following steps,
[0044] A mixture of fluorinated reagents and solvents is provided, and the solvent for the reaction is a high-boiling organic solvent with bp>200°C; raw materials for chloroethylene carbonate are provided;
[0045] The mixture is warmed up, and the chloroethylene carbonate is added dropwise to the mixture when the mixture is warmed up to the required temperature;
[0046] The reaction system is distilled under reduced pressure at the same time as or after the dropwise addition to obtain crude fluoroethylene carbonate.
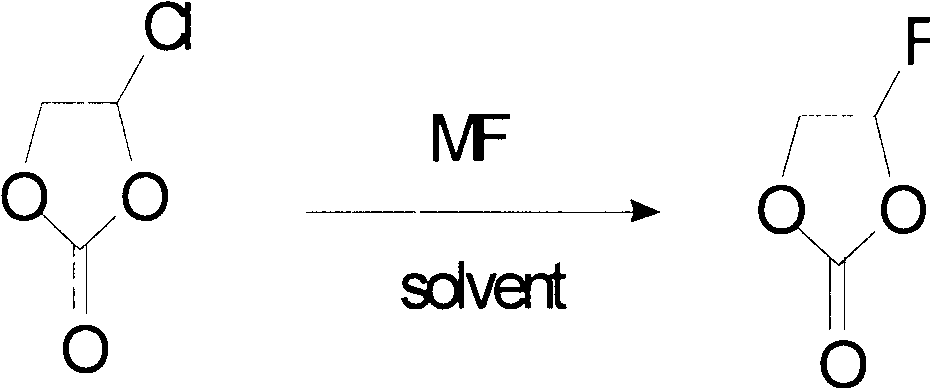
[0047] The method described is as follows:
[0048]
[0049] The "MF" stands for a fluorinating agent; the "solvent" stands for a solvent.
[0050] In a preferred example, the method includes the following steps:
[0051] Add fluorinated reagent and solvent into reactio...
Embodiment 1
[0085] Preparation of Fluoroethylene Carbonate
[0086] Put 4.8 moles of KF and 800 g of tetraethylene glycol dimethyl ether in a 2L reaction flask, heat to an internal temperature of 80-100°C, and dehydrate under reduced pressure until the water content of the solution is less than 50 ppm. Start to add 4.0 moles of chloroethylene carbonate dropwise, and the dropwise addition is completed in about 6 hours. The dropwise addition is carried out with vacuum distillation at the same time, and a total of 600 g of the solution is distilled out, and the reaction conversion rate is 97%. This solution was subjected to rectification under reduced pressure to obtain 381.6 g of fluoroethylene carbonate with a GC content of 99.95%, a yield of 90%, a water content of 8 ppm, and a total chlorine content of 12 ppm.
[0087] MP: 19-20°C.
Embodiment 2
[0089] Preparation of Fluoroethylene Carbonate
[0090] Put 4.8 moles of KF and 800 g of tetraethylene glycol dimethyl ether in a 2L reaction flask, heat to an internal temperature of 80-100°C, and dehydrate under reduced pressure until the water content of the solution is less than 50 ppm. Start to add 4.0 moles of chloroethylene carbonate dropwise, and the dropwise addition is completed in about 6 hours. After the dropwise addition, the stirring reaction is continued for 4 hours, and the reaction conversion rate is 85%. This solution was subjected to rectification under reduced pressure to obtain 326.5 g of fluoroethylene carbonate with a GC content of 99.90%, a yield of 77%, a water content of 12 ppm, and a total chlorine content of 15 ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com