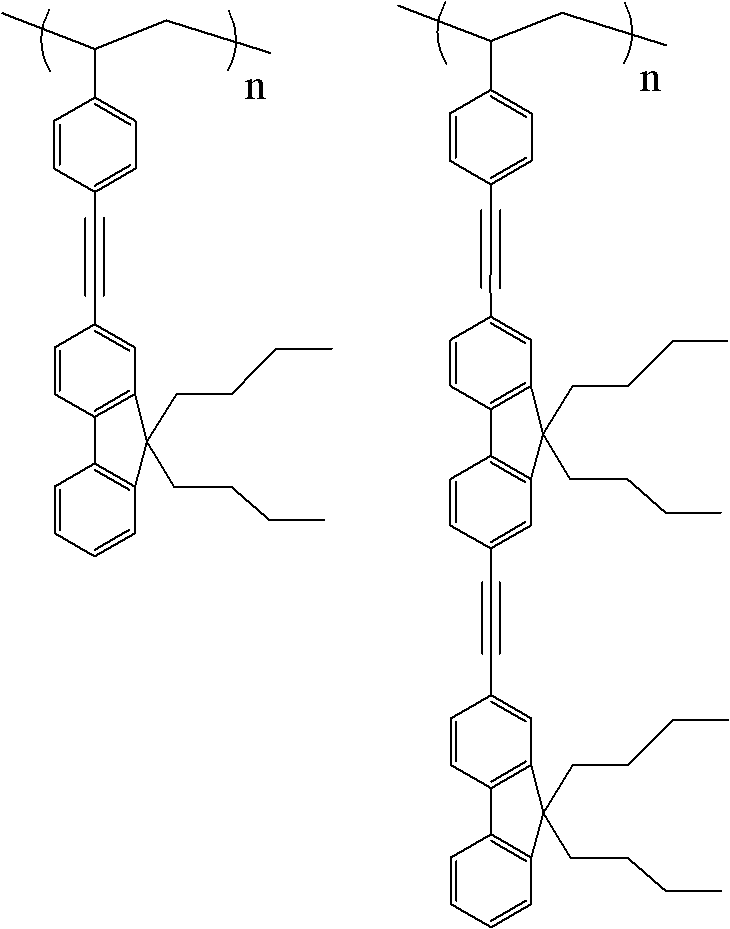
Di-polyfluorene graft polystyrene
A technology of polystyrene and dimeric fluorene, which is applied in the field of oligofluorene grafted polystyrene, can solve the problems of complex synthesis route, high reaction temperature, low total yield and the like, and achieves simple synthesis route and low reaction temperature. , the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
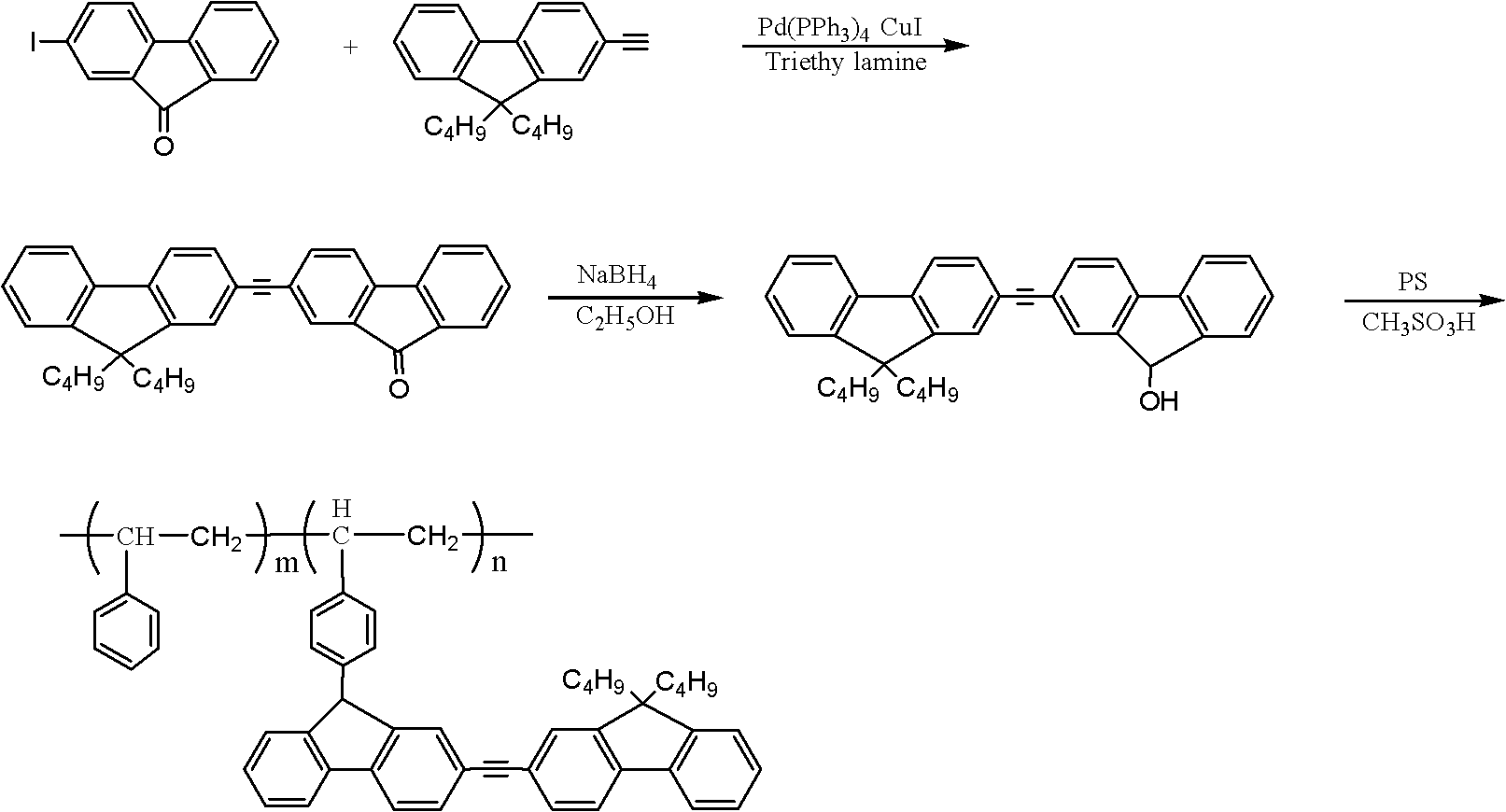
[0020] 1. Synthesis of 2-iodofluorenone: add 20 mmoles of fluorenone, 8 mmoles of iodine powder, 4.8 mmoles of periodic acid, 20 milliliters of acetic acid, 4 milliliters of water in a 50 milliliter round bottom flask, and the concentration of 0.6 milliliters is 98% sulfuric acid. The round bottom flask was placed in a 60°C water bath for 4 hours with constant stirring. After the reaction finished, the mixture in the round bottom flask was poured into the aqueous solution of sodium bisulfite, stirred for 10 minutes, then the solution in the mixture was removed with a suction filter bottle, and the obtained solid was poured into deionized water and washed 3 times. Finally, the solid matter was dried in a vacuum oven, and the obtained light yellow solid was 2-iodofluorenone. Yield 97%.
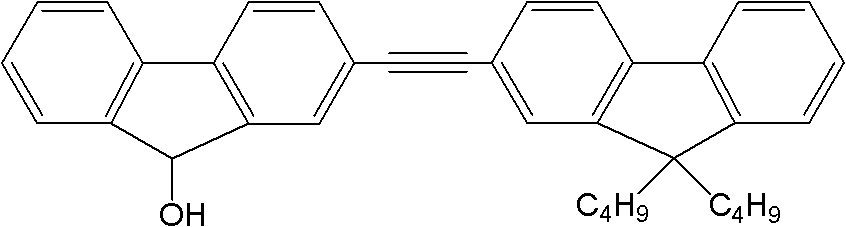
[0021] 2. Synthesis of dipolyfluorene: 3.0 mmol 2-iodofluorenone, 3.0 mmol 9,9-dibutyl-2-(2-methyl-3-butyne-2-ol) fluorene, 0.3 Millimoles of tetrakistriphenylphosphine palladium, 0.3 mmoles ...
Embodiment 2
[0025] 1. Synthesis of 2-iodofluorenone: add 20 mmoles of fluorenone, 8 mmoles of iodine powder, 4.8 mmoles of periodic acid, 20 milliliters of acetic acid, 4 milliliters of water in a 50 milliliter round bottom flask, and the concentration of 0.6 milliliters is 98% sulfuric acid. The round bottom flask was placed in a 60°C water bath for 4 hours with constant stirring. After the reaction finished, the mixture in the round bottom flask was poured into the aqueous solution of sodium bisulfite, stirred for 10 minutes, then the solution in the mixture was removed with a suction filter bottle, and the solid obtained was poured into deionized water and washed 3 times. Finally, the solid matter is dried in a vacuum oven, and the obtained light yellow solid is 2-iodofluorenone. Yield 97%.
[0026]2. Synthesis of dipolyfluorene: 3.0 mmol 2-iodofluorenone, 3.0 mmol 9,9-dibutyl-2-(2-methyl-3-butyne-2-ol) fluorene, 0.3 Add millimole tetrakistriphenylphosphine palladium, 0.3 millimole ...
Embodiment 3
[0030] 1. Synthesis of 2-iodofluorenone: add 20 mmoles of fluorenone, 8 mmoles of iodine powder, 4.8 mmoles of periodic acid, 20 milliliters of acetic acid, 4 milliliters of water in a 50 milliliter round bottom flask, and the concentration of 0.6 milliliters is 98% sulfuric acid. The round bottom flask was placed in a 60°C water bath for 4 hours with constant stirring. After the reaction finished, the mixture in the round bottom flask was poured into the aqueous solution of sodium bisulfite, stirred for 10 minutes, then the solution in the mixture was removed with a suction filter bottle, and the solid obtained was poured into deionized water and washed 3 times. Finally, the solid matter was dried in a vacuum oven, and the obtained light yellow solid was 2-iodofluorenone. Yield 97%.
[0031] 2. Synthesis of dipolyfluorene: 3.0 mmol 2-iodofluorenone, 3.0 mmol 9,9-dibutyl-2-(2-methyl-3-butyne-2-ol) fluorene, 0.3 Add millimole tetrakistriphenylphosphine palladium, 0.3 millimo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com