Treatment of diseases and conditions mediated by eicosanoids
A technology of eicosanoids, diseases, applied in the field of compositions of diseases and disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
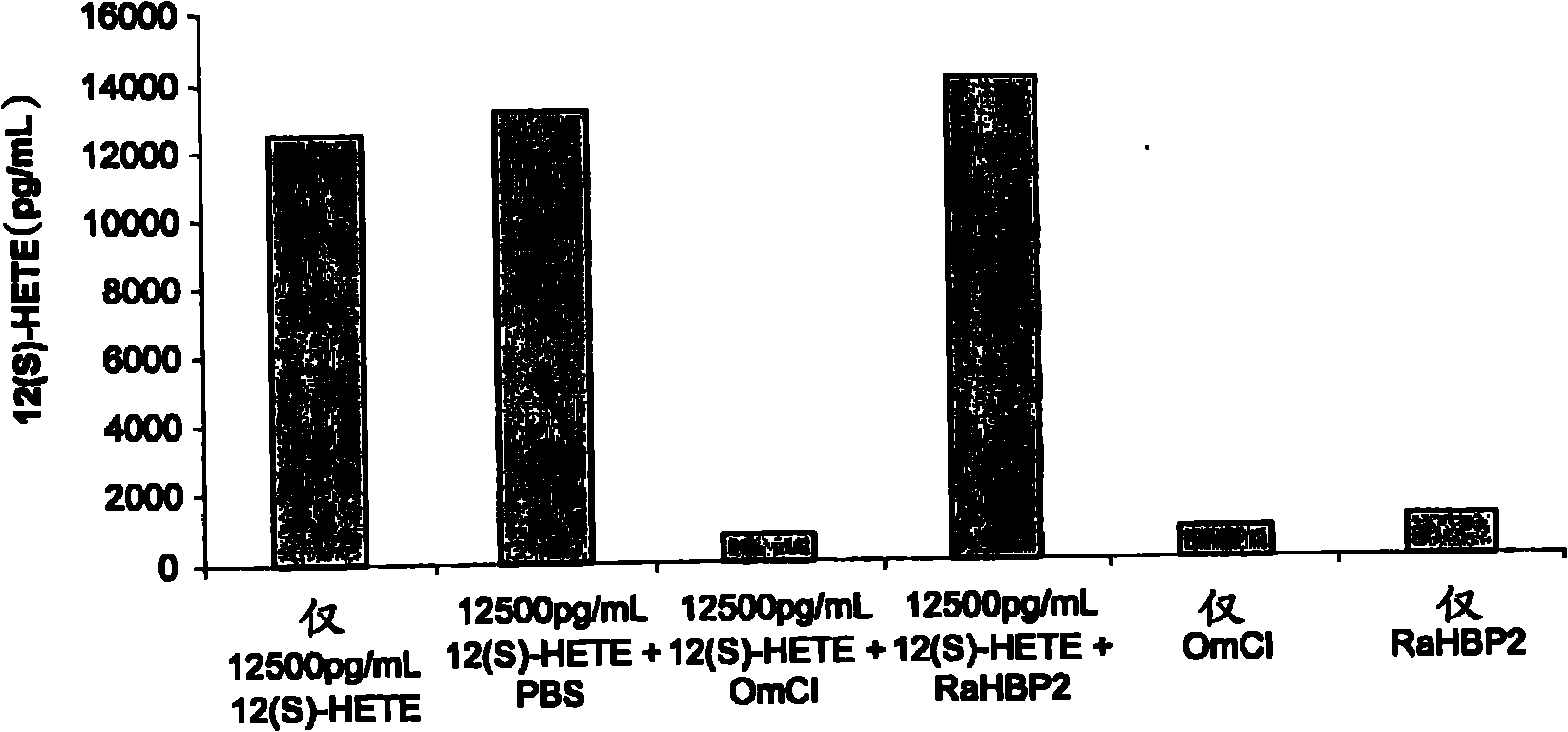
[0118] Example 1: OmCI binding to 12(S)-HETE (12(S)-hydroxyeicosatetraenoic acid) in a competition ELISA
[0119] background:
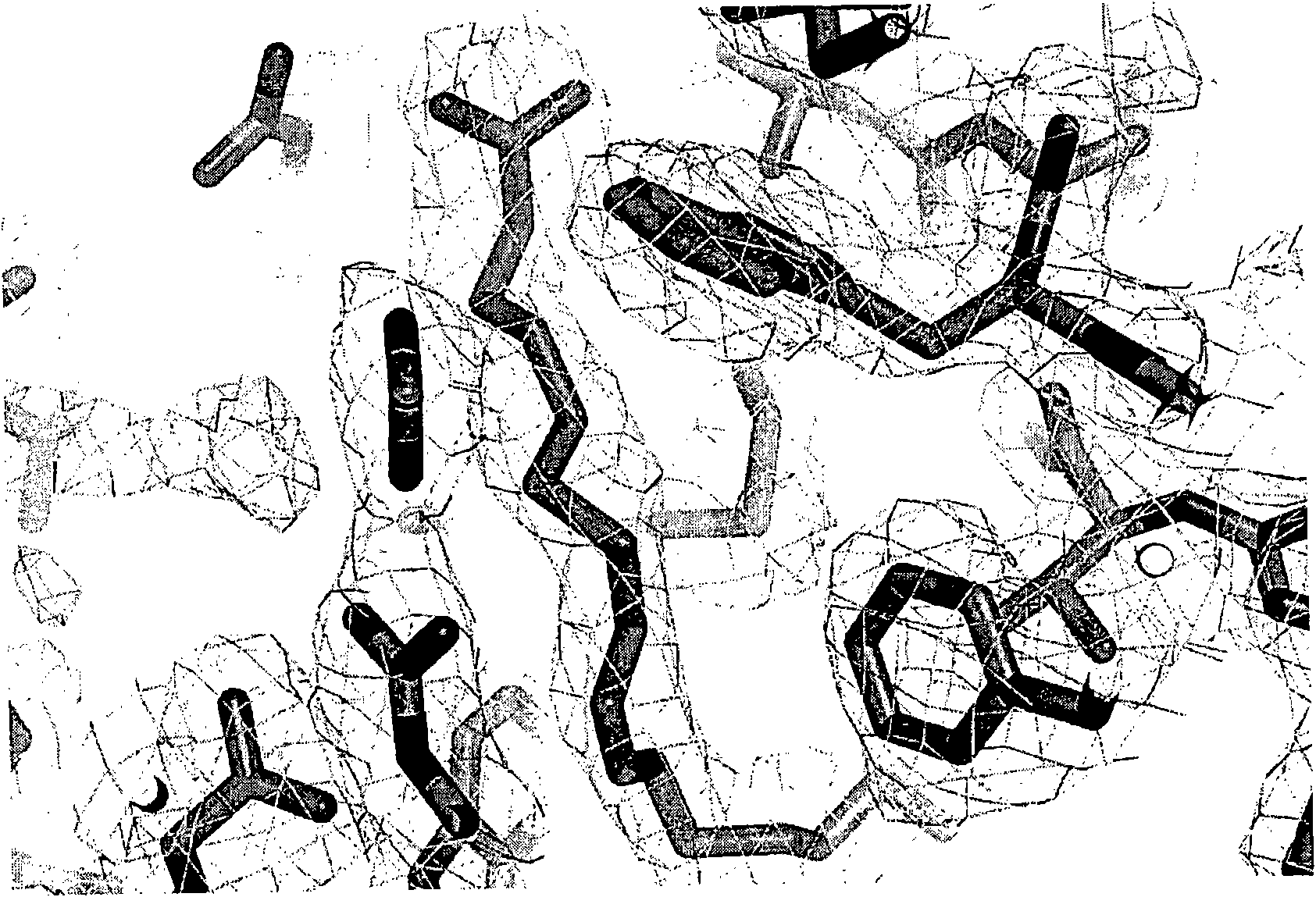
[0120] OmCI binds to fatty acids ( figure 1 ). Mass spectrometry analysis showed that ricinoleic acid (C 18 h 34 o 3 ) and palmitoleic acid (C 16 h 30 o 2 ) are the major forms seen in OmCI expressed in P. methanolica and E. coli, respectively. However, the actual physiological ligands are more likely to be one or more of many eicosanoids derived from host cell membranes that mediate inflammation, oxidative stress, and cell signaling.
[0121] Eicosanoids can be quantified using a competitive enzyme immunoassay (EIA) from Assay Designs Inc. One such EIA kit uses a polyclonal antibody against 12(S)-HETE to compete with 12(S)-HETE labeled with alkaline phosphatase, and unlabeled in samples or standards of known concentration. 12(S)-HETE binding. After simultaneous incubation at room temperature and antibody capture on the plate, excess reagen...
Embodiment 2
[0129] Example 2: Parameters affecting the combination of 12(S)-HETE and OmCI
[0130] method:
[0131] A method similar to that described in Example 1 was used.
[0132] Results and discussion
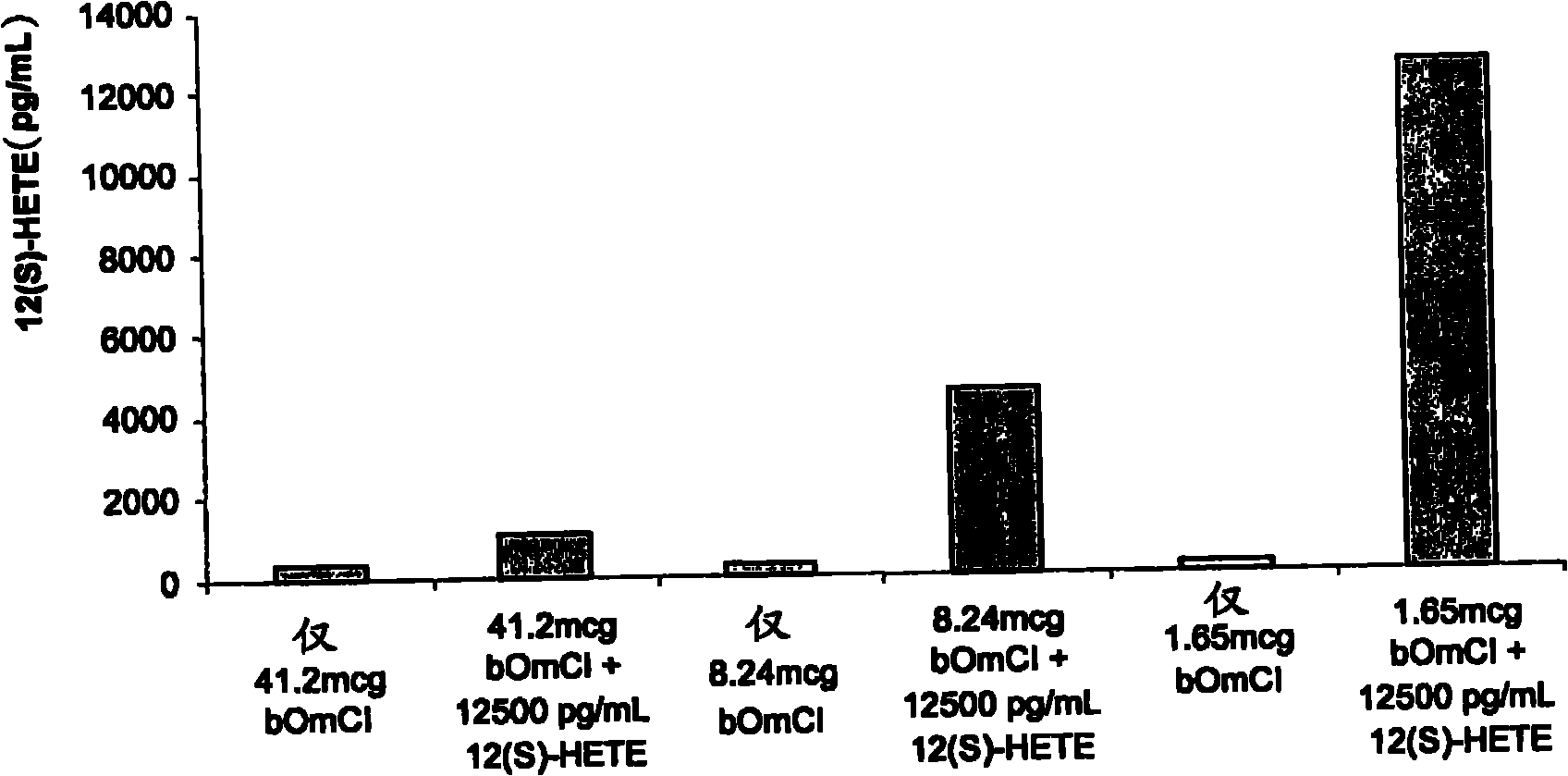
[0133] A significant molar excess of bOmCI to 12(S)-HETE is required to provide results that clearly demonstrate 12(S)-HETE binding ( image 3 ). exist image 3 In the tests shown, bOmCI was in excess of approximately 634, 127 and 25.5 molar relative to 12(S)-HETE. The need for a significant molar excess of OmCI may reflect competition of bOmCI for binding to 12(S)-HETE with anti-12(S)-HETE antibodies, low binding affinity of bOmCI for 12(S)-HETE, and / or binding only to those not protected by palmitate. Oleic acid-occupied bOmCI binding.
[0134] Prolonged incubation (overnight at room temperature) did not increase the proportion of 12(S)-HETE bound to bOmCI ( Figure 4 )
[0135] At equivalent concentrations, yeast (y)OmCI bound less 12(S)-HETE ( Figure 5 ). At a 634 molar...
Embodiment 3
[0137] Example 3: OmCI binds LTB4 but not TXB2 or cysteinyl leukotrienes
[0138] method:
[0139] Buy for Leukotriene B 4 (LTB 4 ), thromboxane B 2 (TXB 2 ) and cysteinyl leukotriene (cys-LK) solution measured Assay Design Inc. EIA kit and used according to product instructions. Mix 100 μl of the standard solution with ≤9 μl of PBS or a diluted stock solution of OmCI or RaHBP2. The mixture was incubated at room temperature for 20 minutes before being used in the immunoassay according to the manufacturer's instructions. The absorbance readings of the treated samples are compared to a standard curve to estimate the concentration of eicosanoid in solution that can be bound by the anti-eicosanoid polyclonal antibody.
[0140] result:
[0141] bOmCI showed no association with the cyclic eicosanoid TXB2 ( Figure 6 ) or amino acid-coupled Cys-LK binding (data not shown). This is in line with our crystallographic data showing that the binding pocket of OmCI is not large eno...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com