Method for preparing n-methylpyrrolidone from 1,4-butanediol
A technology of methylpyrrolidone and butanediol, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Reduce activity and other issues, achieve the effects of inhibiting the formation of by-products, prolonging catalyst life, and improving cost-effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
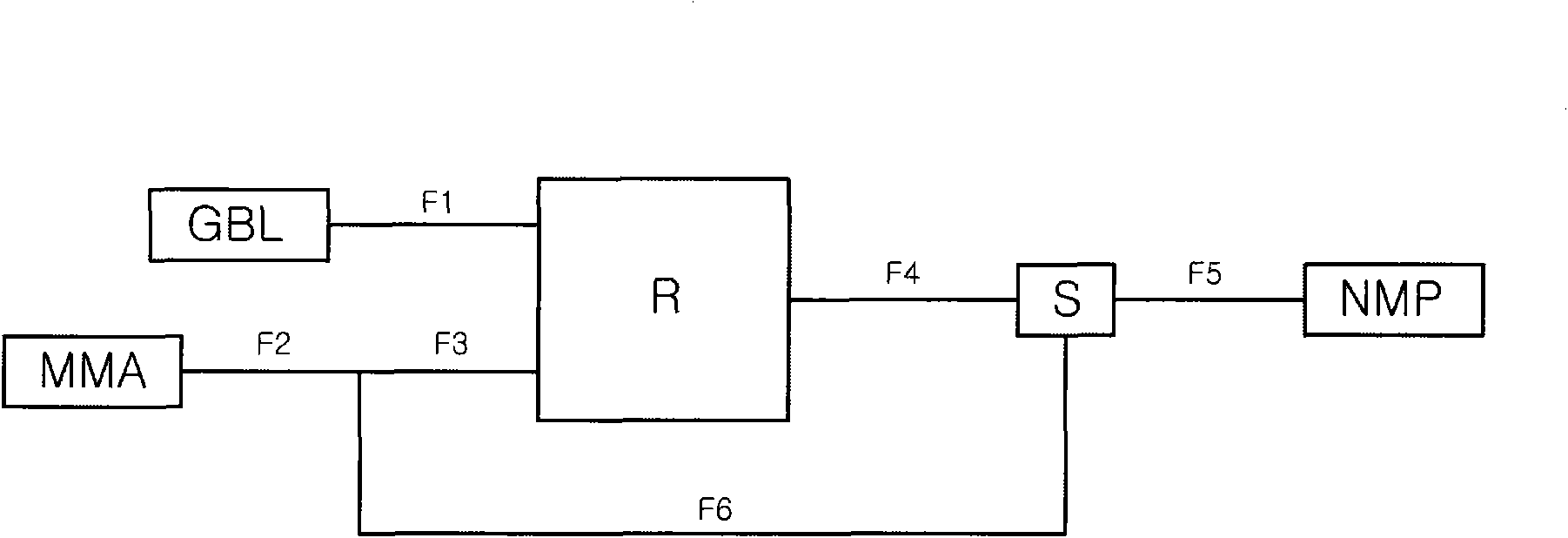
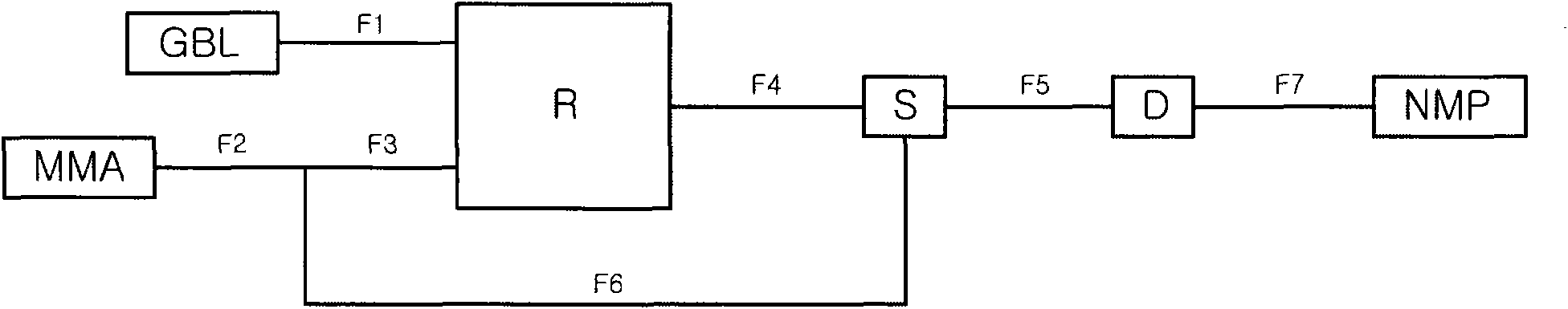
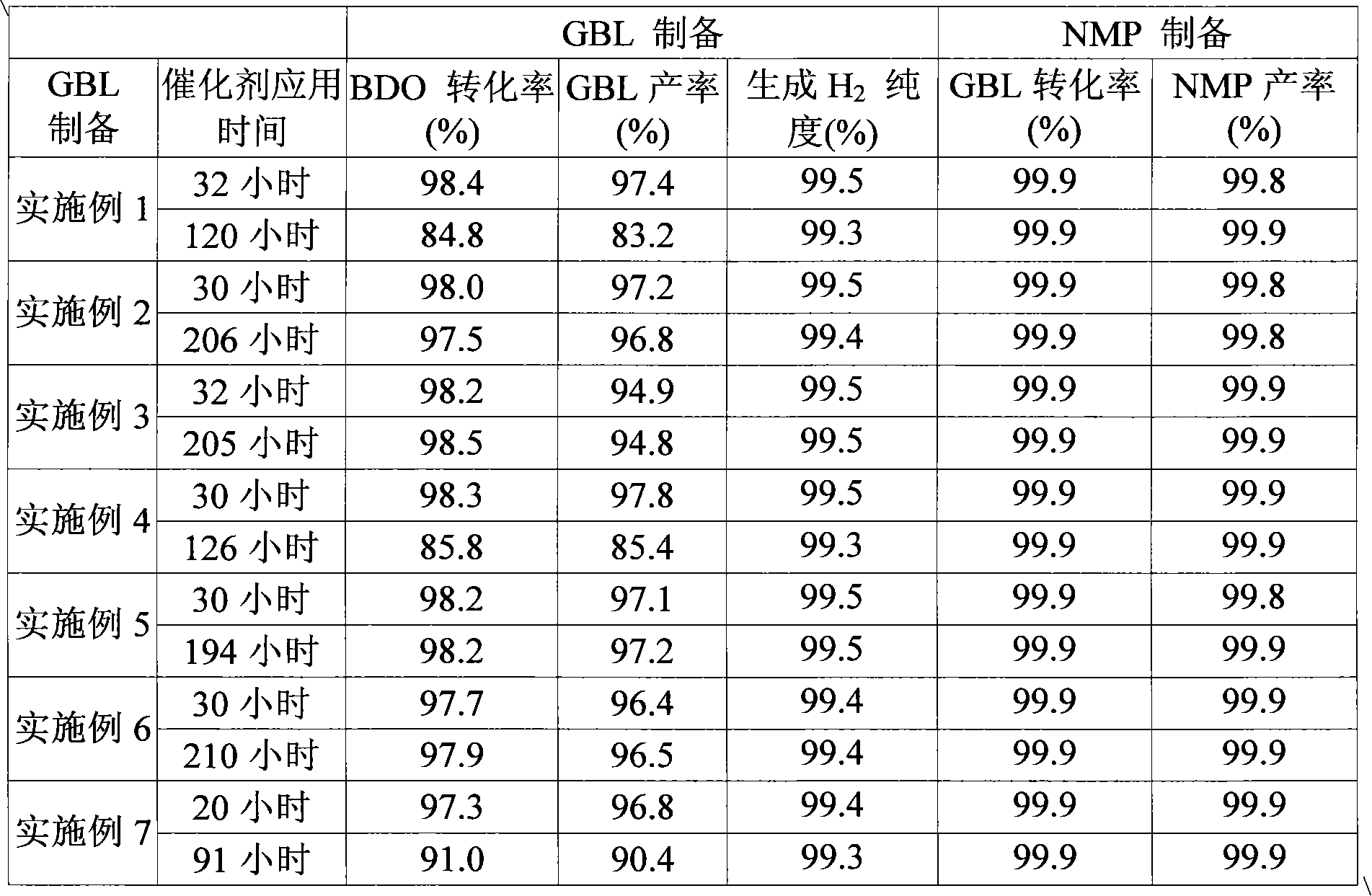
Image
Examples
Embodiment 1
[0049] Copper nitrate, zinc nitrate, and aluminum nitrate were dissolved in distilled water (500 mL) to provide a metal precursor solution, followed by vacuum drying at 70°C. Then, the dry powder was fired at 500° C. for 4 hours in an air atmosphere. Here, each metal nitrate is weighed and dissolved in such a manner that each metal nitrate conforms to the composition of the final metal oxide obtained after firing.
[0050] As a result, a catalyst in the form of an oxide was obtained with the following elemental composition: CuO 51% by weight, ZnO 31% by weight and Al 2 o 3 18% by weight.
[0051] The catalyst (5 g) in the form of the resulting powder was charged into a fixed-bed reactor made of SUS with an inner diameter of 3 / 4 inch. Then, at 4kg / cm 2 The reduction was carried out using hydrogen at a pressure of G and a temperature of 250° C. for 4 hours. After the reduction reaction of the catalyst, at a reaction temperature of 240°C, the weight hourly space velocity (W...
Embodiment 2
[0054] N-Methylpyrrolidone was prepared in the same manner as described in Example 1, except that the final catalyst had a composition of CuO 53 wt%, ZnO 32 wt%, Al 2 o 3 12.5% by weight, Ce 2 o 3 2% by weight and Na 2 O 0.5% by weight mode weighed and used each metal nitrate.
Embodiment 3
[0056] N-Methylpyrrolidone was prepared in the same manner as described in Example 1, except that the final catalyst had a composition of CuO 76% by weight, SiO 2 19.5 wt%, MgO3 wt% and Cr 2 o 3 Each metal nitrate was weighed and used in a manner of 1.5% by weight.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com