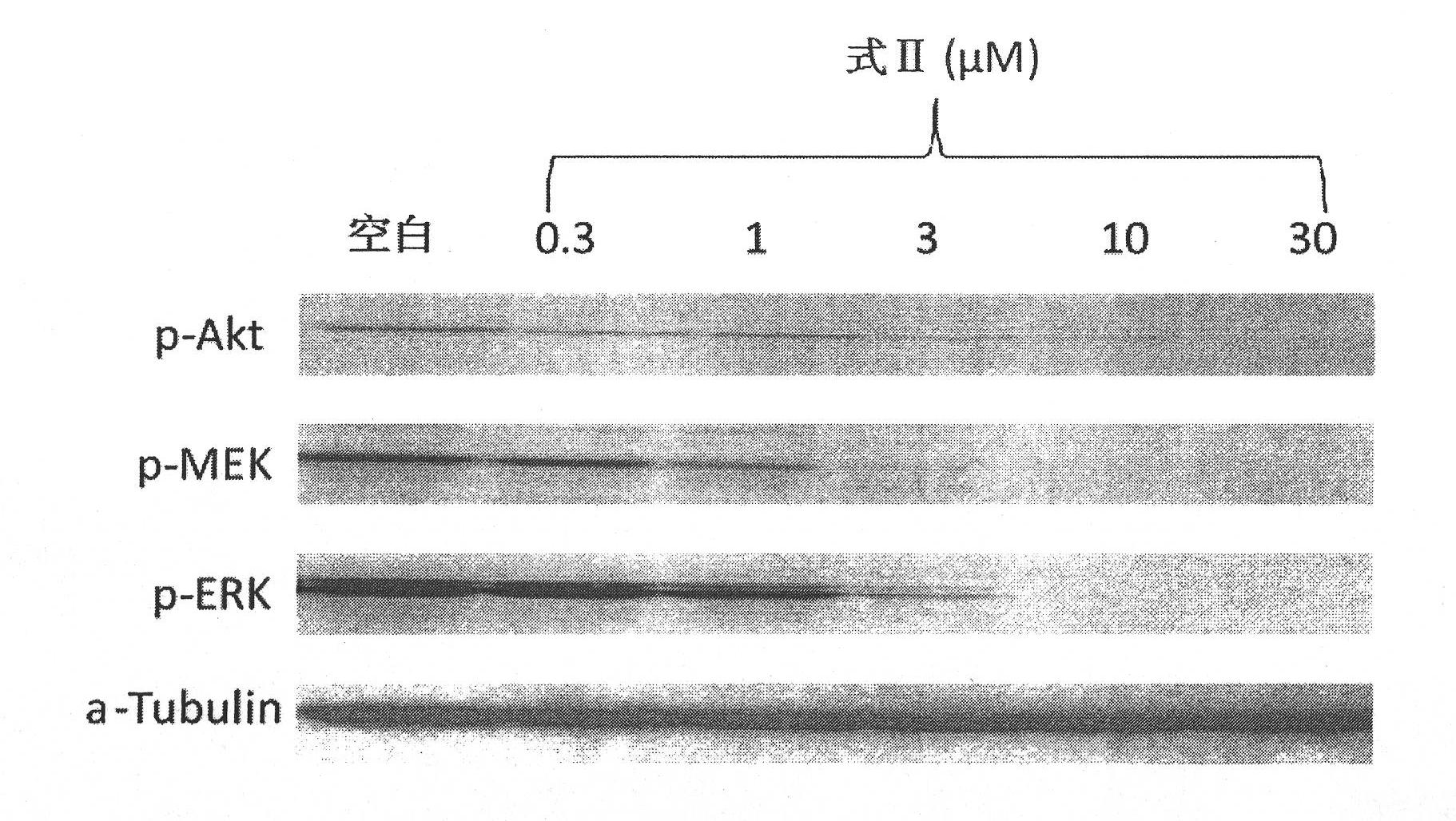
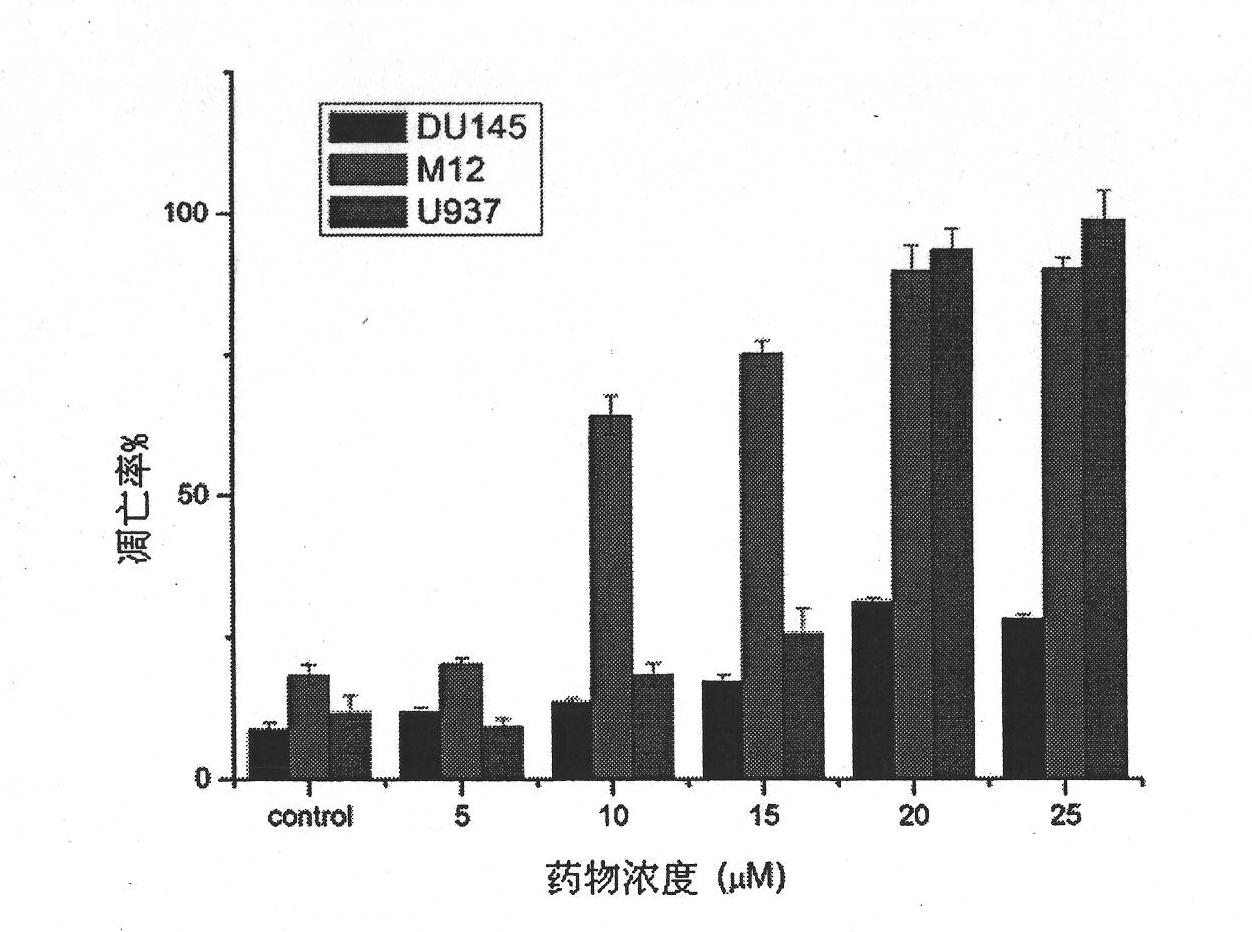
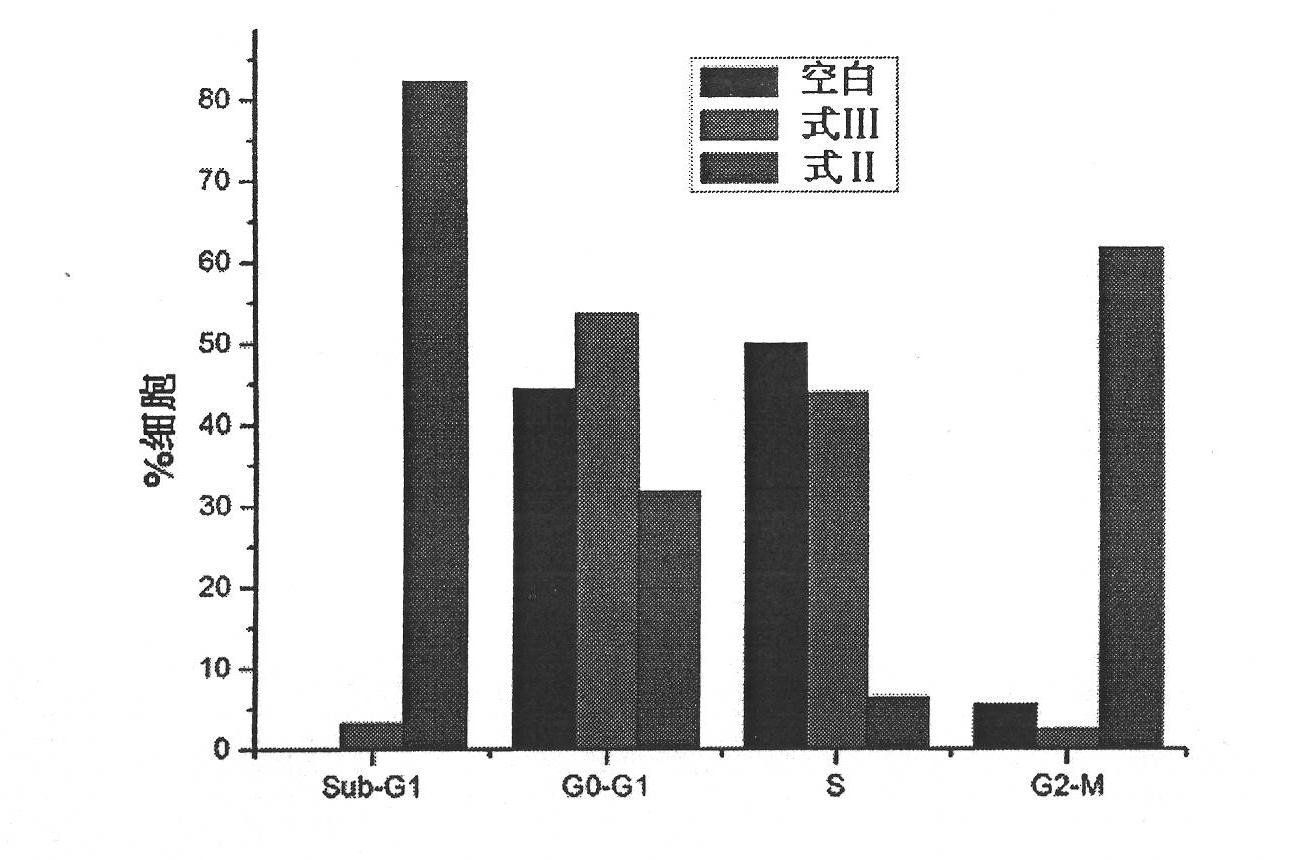
3-(2-amino-ethyl)-5-(3-cyclohexyl-propylidene)-thiazoline-2,4-diketone and derivatives thereof
An amino, C1-C4 technology, used in drug combinations, antipyretics, organic chemistry, etc., can solve problems such as undiscovered monomeric small molecular substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Embodiment 1.N-Boc-bromoethylamine
[0138] Di-tert-butyl dicarbonate (5.85g, 26.8mmol) and triethylamine (3.4mL, 24.4mmol) were dissolved in 25mL of dioxane, and added to 2-bromoethylamine hydrobromide (5.0 g, 24.4mmol) in 50mL dioxane solution. The mixture was stirred and reacted at room temperature for 48 hours, and the precipitate was filtered off. The filtrate was concentrated and dissolved in 100 mL of dichloromethane, washed successively with 0.5N hydrochloric acid, saturated sodium bicarbonate and brine, anhydrous Na 2 SO 4 dry. After distilling off the solvent, N-Boc-bromoethylamine was obtained as a colorless oil. Yield: 89%. 1 H-NMR (300MHz, CDCl 3 ): 3.55-3.52(t, 2H), 3.48-3.44(t, 2H), 1.45(s, 9H).
Embodiment 2
[0139] Example 2. tert-butyl 2-(2,4-dioxthiazol-3-yl)ethylcarbamate
[0140] In the 500mL flask, add 2,4-thiazolidinedione (2.0g, 17.1mmol), K 2 CO 3 (10.6 g, 1.2 e.q), tetrabutylammonium iodide (TBAI, 2.5 g, 0.1 e.q) and 300 mL acetone, and N-Boc-bromoethylamine (11.0 mL, 1.5 e.q) was added. The mixture was refluxed for 10 hours, filtered and evaporated to remove the solvent to obtain a yellow oil, which was dissolved in 100 mL of dichloromethane, washed with brine and washed with anhydrous Na 2 SO 4 dry. The crude product was purified by column chromatography (n-hexane / ethyl acetate=8 / 2) to obtain white crystals. Yield: 80%. 1 H-NMR (300MHz, CDCl 3 ): δ3.96(s, 2H), 3.76(t, 2H), 3.39(t, 2H), 1.43(s, 9H); 13 C-NMR (75MHz, CDCl 3 ): δ173.2, 171.3, 167.4 79.2, 41.6, 37.9, 33.3, 27.9.
Embodiment 3
[0141] Example 3. Cyclohexylpropanal
[0142] Oxalyl chloride (440uL, 5.0mmol) was dissolved in 20mL of dichloromethane, cooled to -78°C, N 2 Pure DMSO (1.0 mL, 14 mmol) was added dropwise at the same temperature under the atmosphere. After 15 minutes, cyclopropyl alcohol (610 μL, 4.0 mmol) was slowly added dropwise under temperature control at -78°C. The reaction was stirred for 1 hour, and the solution gradually became opaque. Add 5.0 mL of triethylamine and slowly warm to room temperature. 20 mL of water was added to separate the layers. The aqueous layer was extracted with 3x20 mL of dichloromethane. The crude product was purified by column chromatography (ethyl acetate / n-hexane=1 / 10). Yield: 89%. 1 H-NMR (400MHz, CDCl 3 ): δ9.77-9.76(t, 1H, J=1.92Hz), 2.45-2.41(dt, 2H, J=7.52, 1.92Hz), 1.71-1.55(m, 5H), 1.51-1.49(q, 2H ), 1.26-1.11(m, 4H), 0.93-0.86(m, 2H); 13 C-NMR (100MHz, CDCl 3 ): 203.1, 63.4, 41.5, 37.5, 37.2, 33.4, 33.0, 30.1, 29.3, 26.7, 26.4, 26.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com