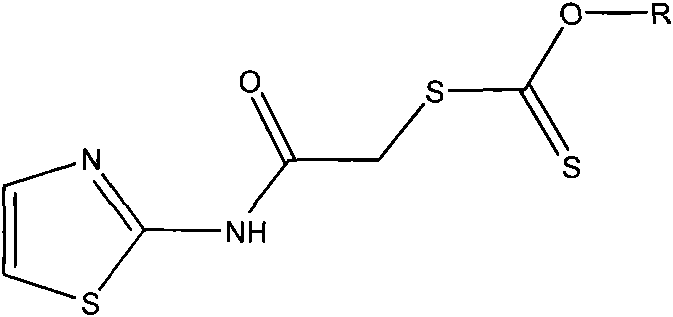
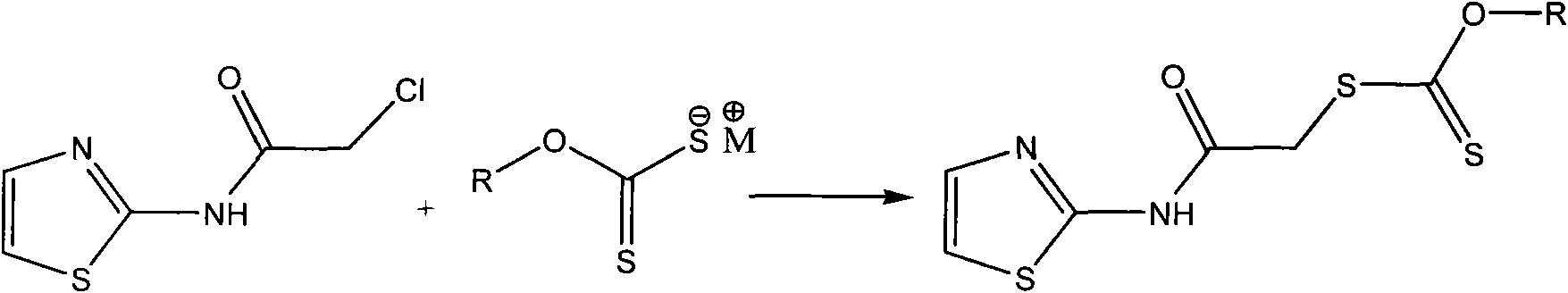
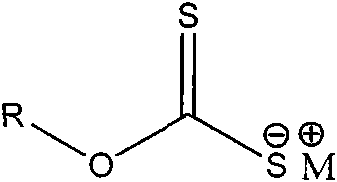
Aminothiazole alkyl xanthate derivative as well as preparation method and application of aminothiazole alkyl xanthate derivative
A technology of aminothiazolidinyl and alkyl xanthate, applied in lubricating compositions, petroleum industry, additives, etc., to achieve excellent extreme pressure and anti-wear performance, mild process conditions, and low synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a 100ml three-necked flask equipped with a thermometer, a stirring device, and a reflux condenser, add 0.012mol of sec-butyl xanthate sodium and 20ml of absolute ethanol, and slowly add 0.01mol of 2-(chloroacetylamino)thiazole dropwise under stirring 80ml of absolute ethanol solution, and reacted at about 10°C for 150min. Then slowly raise the temperature to 70°C. After reacting for 60 minutes, distill off absolute ethanol, add water to dissolve the residue, extract three times with anhydrous ether, and distill the ether solution with anhydrous MgSO 4 Dry for 24h. After filtration, the filtrate was concentrated, cooled, and crystals were precipitated. Recrystallized with acetone to obtain 2-(O-sec-butylxanthyl-S-acetylamino)thiazole as a light yellow solid, weighing 2.64 g, with a yield of 91.0%.
Embodiment 2
[0036] In a 250ml three-necked flask equipped with a thermometer, a stirring device, and a reflux condenser, add 0.015mol of sodium n-pentyl xanthate and 100ml of acetone, and slowly add 0.01mol of 2-(chloroacetamido)thiazole in acetone solution dropwise under stirring 50ml, react at a constant temperature of about 10°C for 150min. Then slowly raise the temperature to 70°C. After reacting for 60 minutes, distill off the acetone, add water to dissolve the residue, extract 3 times with diethyl ether, and distill the diethyl ether solution with anhydrous MgSO 4 Dry for 24h. After filtration, the filtrate was concentrated, cooled, and crystals were precipitated. Recrystallized with absolute ethanol to obtain light yellow solid 2-(O-n-pentylxanthogen-S-acetylamino)thiazole, weighing 2.71 g, yield 89.1%.
Embodiment 3
[0038] In a 250ml three-neck flask equipped with a thermometer, a stirring device, and a reflux condenser, add 0.013mol of isooctyl xanthate sodium and 100ml of tetrahydrofuran, and slowly add 0.01mol of 2-(chloroacetylamino)thiazole in tetrahydrofuran under stirring. The solution was 50ml, and reacted at a constant temperature of about 10°C for 150min. Then slowly raise the temperature to 70°C. After reacting for 60 minutes, evaporate the tetrahydrofuran, add water to dissolve the residue, extract 3 times with diethyl ether, and distill the diethyl ether solution with anhydrous MgSO 4 Dry for 24h. After filtration, the filtrate was concentrated, cooled, and crystals were precipitated. Recrystallized with acetone to obtain light yellow solid 2-(O-isooctylxanthogen-S-acetylamino)thiazole, weighing 3.05 g, yield 88.1%.
[0039] The structure of the product obtained in the above examples was confirmed by C, H, N elemental analysis, ultraviolet spectrum, infrared spectrum, hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com