SiRNA for inhibiting canine p53 gene expression and canine cell model of p53 gene silence
A p53 gene and cell model technology, applied in DNA/RNA fragments, cells modified by introducing foreign genetic material, gene therapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The sequence design of embodiment 1 canine p53siRNA
[0044] The gene sequence of the canine p53 gene was obtained from GenBank (accession number: AF060514.1). The siRNA sequences that may be designed to inhibit the expression of canine p53 gene are as follows:
[0045] 5'-GCCACUAGAUGGAGAAUAU-3' (SEQ ID No.1);
[0046] 3'-CGGUGAUCUACCUCUUAUA-5' (SEQ ID No. 2).
[0047] The target sequence of the canine p53 siRNA interference is located at positions 927-945 of the canine p53 gene sequence (see figure 1 ). The target sequence of the dog p53 siRNA (DNA encoding the siRNA of the present invention) is as follows:
[0048] Sense strand: 5'-GCCACTAGATGGAGAATAT-3' (SEQ ID No.3);
[0049] Antisense strand: 3'-CGGTGATCTACCTCTTATA-5' (SEQ ID No. 4).
[0050] And choose a random sequence as a control. The control sequence is as follows:
[0051] Sense strand: 5'-TTCTCCGAACGTGTCACGT-3';
[0052] Antisense strand: 3'-AAGAGGCTTGCACAGTGCA-5'.
[0053] The above-mentioned sens...
Embodiment 2
[0054] Example 2 Packaging of canine p53 siRNA lentivirus
[0055] The above synthesized DNA fragment encoding the siRNA of the present invention and the reference sequence were packaged into lentiviral vectors by Jikai Gene Chemical Co., Ltd. The DNA fragment was ligated into the pGC-LV plasmid. Positive clones were screened by PCR and identified by sequencing. Then, the resulting pGC-LV recombinant plasmid, pHelper 1.0 plasmid and pHelper 2.0 plasmid were co-transfected into 293T cells, cultured for 48 hours, packaged to produce lentivirus, and the cell supernatant was collected. The cell supernatant contains lentiviral particles packaged with the siRNA fragments of the present invention, which are concentrated to obtain a high-titer lentiviral concentrate.
[0056] Viral titers of recombinant lentiviruses were determined using 293T cells. The viral titer of the lentivirus containing the siRNA sequence of the present invention is 10 8 TU / mL, the viral titer of the lentiv...
Embodiment 3
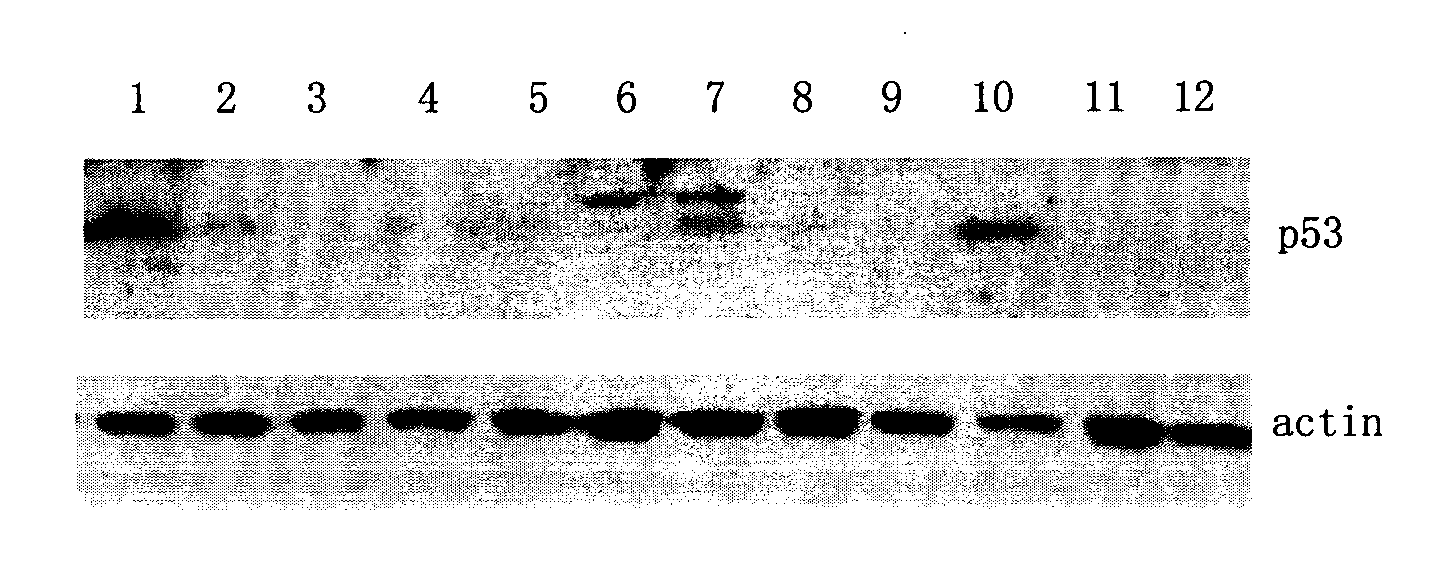
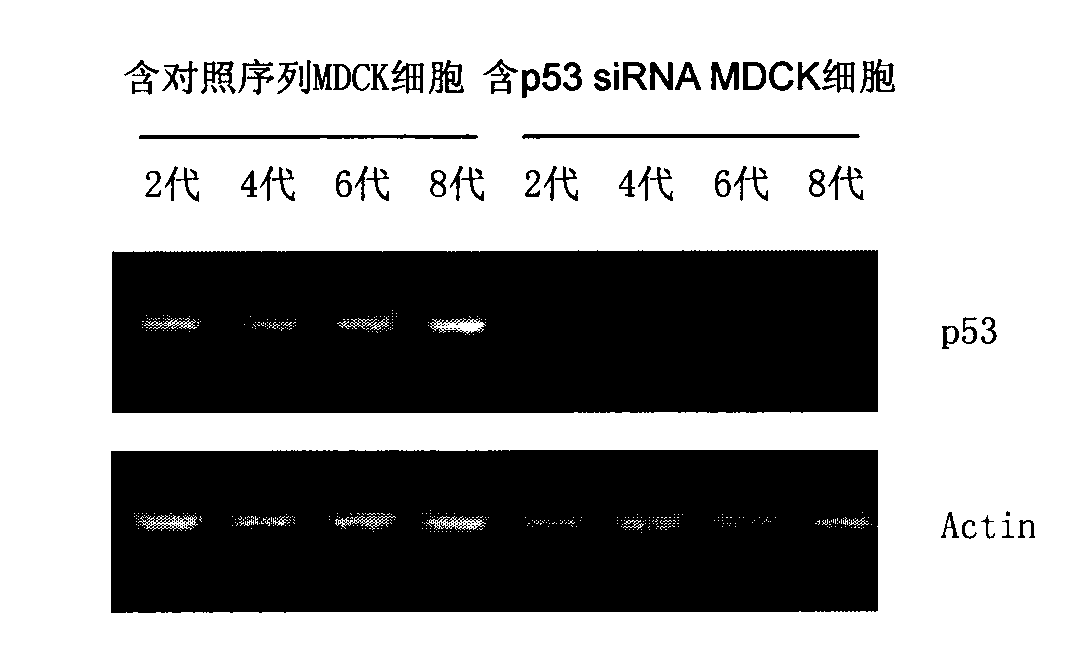
[0057] Example 3 Screening of MDCK cell clones containing canine p53siRNA and analysis of p53 gene expression
[0058] A. On the day before the test, put 5×10 4 MDCK cells (ATCC, USA) were inoculated into three 35mmdish (cell culture dishes).
[0059] B. When the cells grow to about 30-50% cell density, aspirate the supernatant and wash twice with PBS. The MDCK cells were respectively infected with the lentivirus containing the siRNA sequence of the present invention and the lentivirus containing the control sequence, and the virus infection amount was 20 MOI. MDCK cells not infected with lentivirus were set as the control group. Add DMEM medium containing 10% (v / v) serum, at 37°C, 5% CO 2 Continue culturing in the cell culture incubator.
[0060]C. After culturing for 24 hours, remove the medium, add DMEM medium containing 10 μg / ml Puromycin (puromycin) and 10% serum, and continue culturing. Observe the growth state of the cells. When the cells in the control group of MD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com