Synthesis method of 2,2'-diaryl-4,4',5,5'-biphenyl tetraacid dianhydride monomer
A technology of biphenyltetracarboxylic dianhydride and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of low yield and difficult separation of products, and achieve the reduction of dielectric constant, improvement of solubility and light transmittance, and excellent thermal conductivity. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
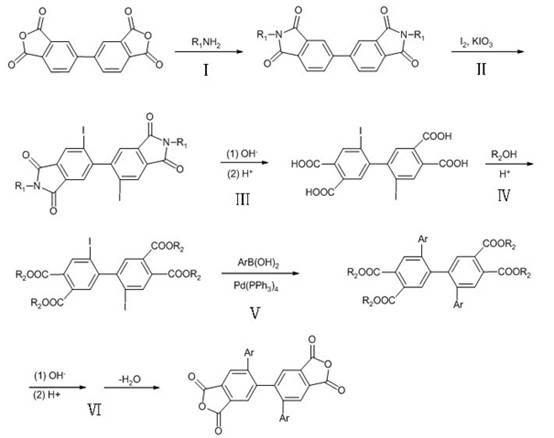
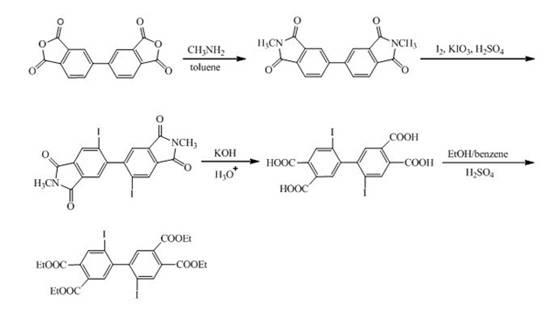
[0032] Synthesis of 2,2'-diphenyl-4,4',5,5'-biphenyltetraacid dianhydride
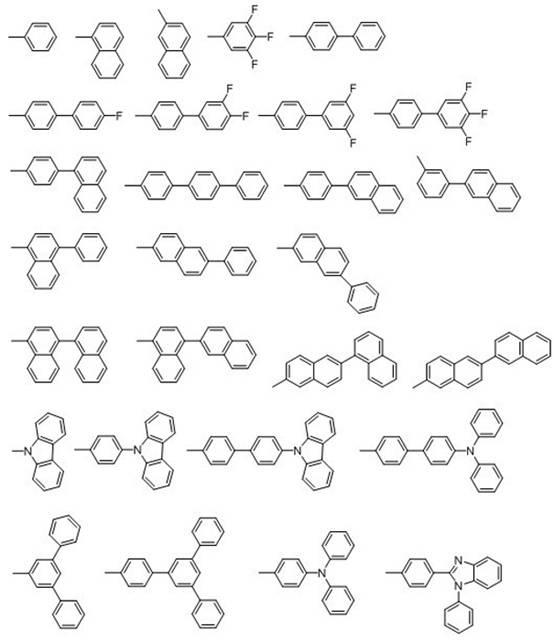
[0033] 2,2'-Diiodo-4,4',5,5'-biphenyl tetraethyl ester (40.0 g, 0.058 mol), phenylboronic acid (21.08 g, 0.17 mol), Pd (PPh 3 ) 4 (1.60 g), Na 2 CO 3 (18.29 g), toluene (287 mL), H 2 O (154 mL) on N 2 Under protection, heat to 70 °C. After reacting for 4 h, the toluene layer was dark red, filtered and separated. The toluene layer was evaporated to dryness to obtain dark red viscous liquid, which solidified after cooling. Recrystallized with ethanol to obtain 25.04 g of white solid, yield: 73.2%. Measured melting point: 159.5-162.6 °C. FI-TR (KBr, cm -1 ): 1724 (C=O), 1242 (C–O–C), 1136 (C–O–C), 697 (Ar–H). 1 H NMR (300 MHz, DMSO-d 6 , ppm) 7.84 (s, 1H, Ar–H), 7.46 (s, 1H, Ar–H), 7.22 (t, 1H, J =14.72 Hz, Ar-H), 7.11 (t, 2H, J =14.92 Hz, Ar-H), 6.56 (d, 2H, J =8.43 Hz, Ar-H), 4.25-4.34 (m, 4H, CH 2 ), 1.25-1.32 (m, 6H, CH 3 ). Elemental Analysis: C 36 h 34 o 8 (594.65). Theoretical ...
Embodiment 2
[0037] Synthesis of 2,2'-bis(4''-phenyl)phenyl-4,4',5,5'-biphenyltetraacid dianhydride
[0038] 2,2'-Diiodo-4,4',5,5'-biphenyl tetraethyl ester (40.0 g, 0.058 mol), 4'-biphenylboronic acid (31.68 g, 0.16 mol), Pd(PPh 3 ) 4 (1.60 g), Na 2 CO 3 (18.29 g), toluene (287 mL), H 2 O (154 mL) on N 2 Under protection, heat to 70 °C. After reacting for 4 h, the toluene layer was dark red, filtered and separated. The toluene layer was evaporated to dryness to obtain dark red viscous liquid, which solidified after cooling. Recrystallized with ethanol to obtain 30.95 g of white solid, yield: 71.6%. Measured melting point: 172-173 °C. FI-TR (KBr, cm -1 ): 1724 (C=O), 1243 (C–O–C), 1136 (C–O–C, 765), 697 (Ar–H). 1 H NMR (300 MHz, DMSO-d 6 , ppm) 7.90 (s, 1H, Ar–H), 7.65 (d, 2H, J =7.26 Hz, Ar-H), 7.53 (s, 1H, Ar–H), 7.37-7.49 (m, 5H, Ar–H), 7.66 (d, 2H, J =8.24Hz, Ar-H), 4.25-4.35 (m, 4H, CH 2 ), 1.25-1.33 (m, 6H, CH 3 ). Elemental Analysis: C 48 h 42 o 8 (746.84). Theor...
Embodiment 3
[0042] Synthesis of 2,2'-bis(4''-(α-naphthyl)phenyl)-4,4',5,5'-biphenyltetraacid dianhydride
[0043] 2,2'-Diiodo-4,4',5,5'-biphenyl tetraethyl ester (40.0 g, 0.058 mol), 4'-(α-naphthyl)phenylboronic acid (32.25 g, 0.13 mol) , Pd(PPh 3 ) 4 (1.60g), K 2 CO 3 (23.81 g), toluene (250 mL), H 2 O (150 mL), on N 2 Under protection, heat to 110 ℃. After reacting for 4 h, the toluene layer was dark red, filtered and separated. The toluene layer was evaporated to dryness to obtain dark red viscous liquid, which solidified after cooling. Recrystallized with ethanol to obtain 25.04 g of white solid, yield: 69.5%. Measured melting point: 179-180°C. FT-IR (KBr, cm -1 ): 1726 (C=O), 1292 (C–O–C), 1136 (C–O–C), 780 (Ar–H). 1 HNMR (300 MHz, DMSO-d 6 , ppm) 7.99 (s, 1H, Ar–H), 1.83-7.94 (m, 3H, Ar–H), 7.68 (s, 1H, Ar–H), 7.35-7.53 (m, 4H, Ar–H) , 7.26 (d, 2H, J =7.89 Hz, Ar–H), 6.79 (d, 2H, J =8.13 Hz, Ar–H), 4.36-4.48 (m, 4H, CH 2 ), 1.36-1.48 (m, 6H, CH 3 ). Elemental Analy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com