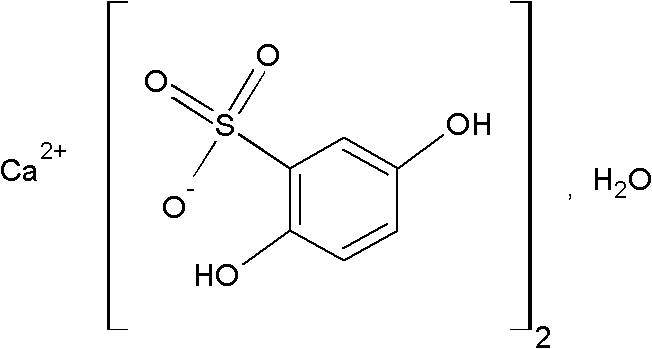
Calcium dobesilate capsule and preparation method thereof
A technology of calcium dobesilate and capsules, which is applied in the directions of capsule delivery, pharmaceutical formulations, and medical preparations with inactive ingredients, etc., can solve problems such as non-conformity of product dissolution, sticking of calculators, and inability of production to proceed normally.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121]
[0122] The capsules are prepared as follows:
[0123] (1) Weigh each raw and auxiliary material, pass calcium dobesilate and sodium hydroxymethylcellulose through a 100-mesh sieve, and dry at 60±5°C for 1 hour;
[0124] (2) dissolving 2.5-5g polyvinylpyrrolidone with ethanol to prepare adhesive;
[0125] (3) Get 8 / 13 of the binder, add 55% calcium dobesilate and 60% croscarmellose sodium of the prescription amount to it, mix, and make primary granules;
[0126] (4) Put the prepared primary granules into a fluidized bed, add 5 / 13 binding agent and calcium dobesilate of 45% of recipe quantity, croscarmellose sodium of 40% recipe quantity, and further prepare granules until there is no remaining powder, and the whole granules are intermediate granules;
[0127] (5) Add the ethanol solution of magnesium stearate to the intermediate granule spray, mix well, dry, detect the intermediate, fill it in 0# empty capsules after calculating the loading according to the interm...
Embodiment 2
[0129]
[0130] The capsules are prepared as follows:
[0131] (1) Weigh each raw and auxiliary material, pass calcium dobesilate and sodium hydroxymethylcellulose through a 100-mesh sieve, and dry at 65°C for 1 hour;
[0132] (2) dissolving 2.5-5g polyvinylpyrrolidone with ethanol to prepare adhesive;
[0133] (3) Get 8 / 13 of the binder, add 60% calcium dobesilate and 70% croscarmellose sodium of the prescription amount to it, mix, and make primary granules;
[0134] (4) Put the prepared primary granules into a fluidized bed, add 5 / 13 binder and calcium dobesilate of 40% of prescription quantity, croscarmellose sodium of 30% of prescription quantity, and further prepare granules until there is no remaining powder, and the whole granules are intermediate granules;
[0135] (5) Add the ethanol solution of magnesium stearate to the intermediate granule spray, mix well, dry, detect the intermediate, fill in 0# empty capsules after calculating the loading according to the int...
Embodiment 3
[0137]
[0138] The capsules are prepared as follows:
[0139] (1) Weigh each raw and auxiliary material, pass calcium dobesilate and sodium hydroxymethylcellulose through a 100-mesh sieve, and dry at 63°C for 1 hour;
[0140] (2) dissolving 2.5-5g polyvinylpyrrolidone with ethanol to prepare adhesive;
[0141](3) Get 8 / 13 of the binder, add 50% calcium dobesilate and 55% croscarmellose sodium of the prescription amount to it, mix, and make primary granules;
[0142] (4) Put the prepared primary granules into a fluidized bed, add 5 / 13 binder and calcium dobesilate of 50% of recipe quantity, croscarmellose sodium of 45% recipe quantity, and further prepare granules until there is no remaining powder, and the whole granules are intermediate granules;
[0143] (5) Add the ethanol solution of magnesium stearate to the intermediate granule spray, mix well, dry, detect the intermediate, fill in 0# empty capsules after calculating the loading according to the intermediate detect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com