Application of bacillus coagulans in preparing medicine for treating inflammatory bowel disease
A technology for Bacillus coagulans and inflammatory bowel disease, applied in the field of biological medicine, can solve the problems of low curative effect, secondary infection, large adverse reactions, etc., to enhance mucosal immune function, prevent the recurrence of inflammation, and improve disease resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0021] Pharmaceutical Preparation Example 1. Preparation of live Clostridium butyricum bacteria powder:
[0022] Take a tube of live Clostridium butyricum strain and dissolve it in a sterilized 100ml Erlenmeyer flask containing 10ml of saline and glass beads. Activate for 10 minutes. Use a 1ml sterile straw to inoculate 1ml of bacterial suspension and inoculate a 50ml flask. In a 250ml Erlenmeyer flask with enrichment medium, place it in a rocking bed at 37°C with constant temperature shaking (190rpm) for 24 hours, transfer to a 2500ml baffle flask containing 450ml of amplification medium, culture at 37°C with constant temperature shaking for 24 hours, microscopic examination After there is no bacteria, transfer to the seed tank with 4.5L expansion medium, anaerobic culture (aeration volume 3:1) for 24 hours, and then transfer to the fermentation tank with 45L fermentation medium after microscopic inspection without bacteria During anaerobic culture at 37°C for 24 hours, the spor...
preparation Embodiment 2
[0023] Pharmaceutical preparation example 2. Preparation of live Clostridium butyricum capsules
[0024] Composition% (weight)
[0025] Clostridium butyricum live bacteria powder 20.00 copies
[0026] Microcrystalline cellulose 40.00 parts
[0027] Glucose 40.00 parts
[0028] Mix well in groups 1 to 3 of the above components, and then use conventional capsule filling technology to form capsules according to the unit dose.
experiment example 1
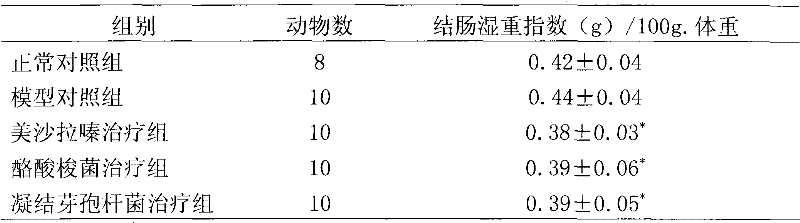
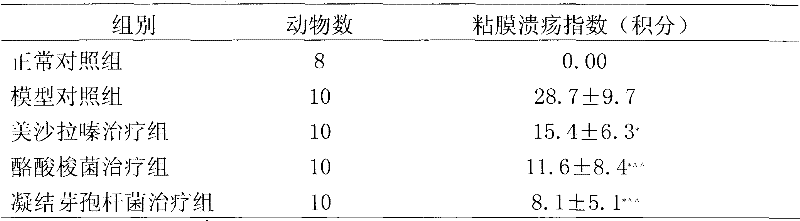
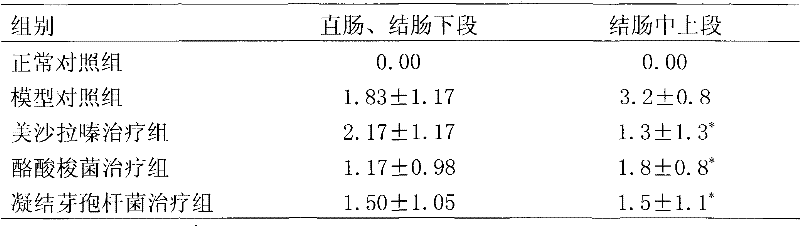
[0029] Basic experimental example 1. Therapeutic effect of Clostridium butyricum and Bacillus coagulans on immune rats with inflammatory bowel disease
[0030] 1 Experimental materials and methods
[0031] 1.1 Experimental animals: Male SD rats, 180-220g, purchased from Weitong Lihua Experimental Animal Center, clean grade, certificate number: SCXP (Beijing) (2002)-0003.
[0032] 1.2 Experimental drugs: Mesalazine (200mg / ml); Clostridium butyricum (CGMCC No.0313.1 strain, 10 8 CFU / ml), Bacillus coagulans (CGMCC No. 1207 strain, 10 8 CFU / ml) was provided by Qingdao Donghai Pharmaceutical Co., Ltd.
[0033] 1.3 Experimental reagents: Freund's complete adjuvant (sigma), MTT (sigma), concanavalin (ConA) (Sigma); lipopolysaccharide (LPS) (Sigma); MTT (Sigma), purchased from Beijing Shubowei Chemical Instrument Co., Ltd.; 1640 culture medium (Gibco); bovine colon mucosal protein freeze-dried powder (homemade); IL-8 ELISA kit (BD); TNFα ELISA kit (Ebioscience); rat IgG reference serum; rabbi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com