Method for preparing high-purity manganese nitrate and co-generating gypsum by using waste sulfuric acid treated by rhodochrosite
A technology of combining manganese nitrate and waste sulfuric acid, applied in the directions of manganese nitrate, calcium/strontium/barium sulfate, etc., can solve the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
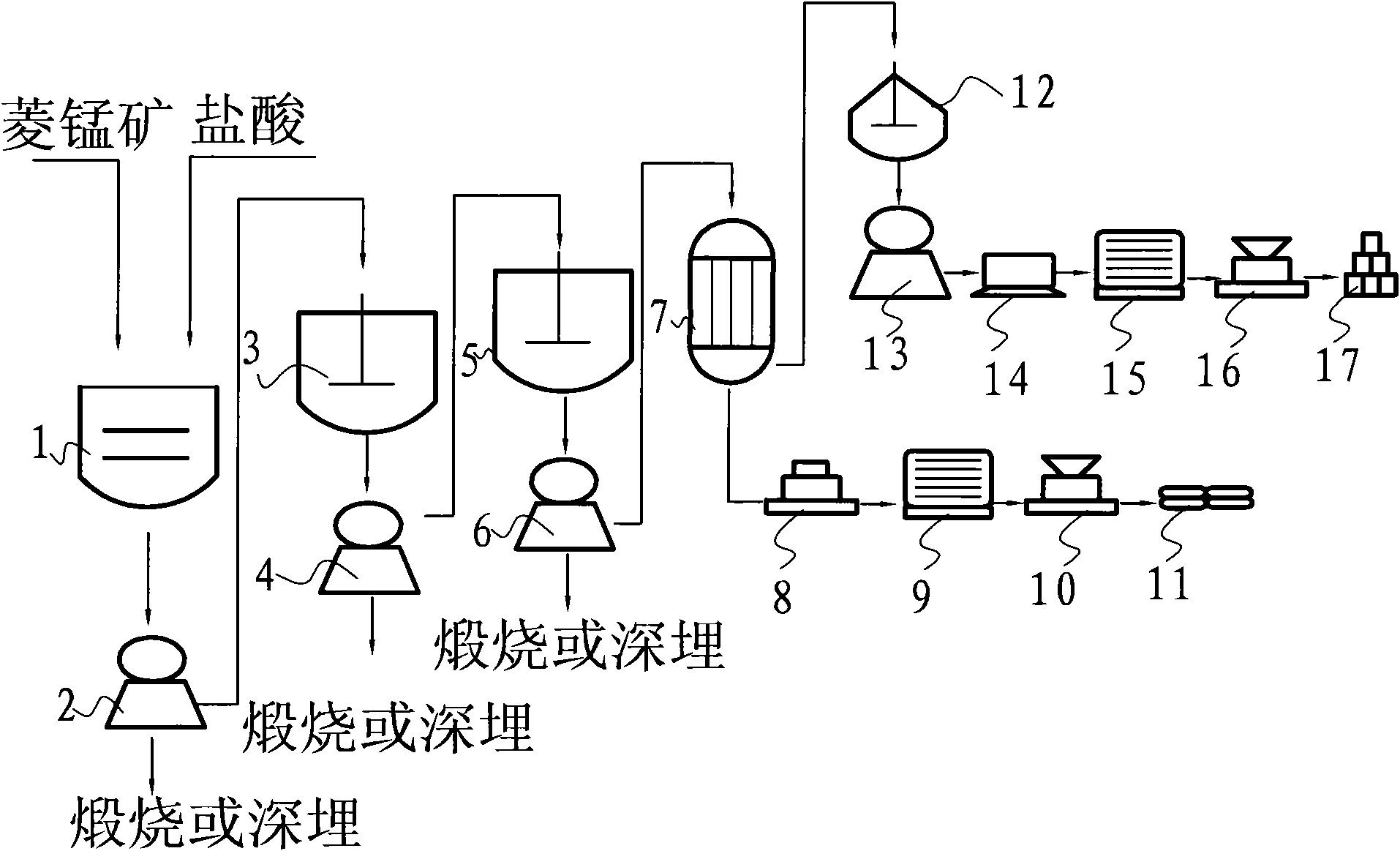
[0034] Using rhodochrosite to process waste sulfuric acid to prepare high-purity manganese nitrate and produce gypsum, the preparation steps are as follows:
[0035] Step A, 800kg rhodochrosite is pulverized into 60 purpose coarse powder;
[0036] Step B, putting the rhodochrosite coarse powder into the leaching tank 1 equipped with hydrochloric acid, and leaching for 6 hours with a pure mass ratio of 1:0.43; The concentration is 15%;
[0037] Step C, filtering the solid-liquid mixture leached in step B with the first filter 2, and calcining or deeply burying the filter cake impurities;
[0038] Step D, the filtrate obtained by filtering in step C and sulfuric acid are successively dropped into the first anticorrosion reactor 3 with a pure mass ratio of 1: 0.58 to carry out stirring reaction, and the pure mass ratio of the filtrate to calcium hydroxide is according to the pure manganese chloride in the filtrate and sulfuric acid for conversion; before the filtrate is reacted...
Embodiment 2
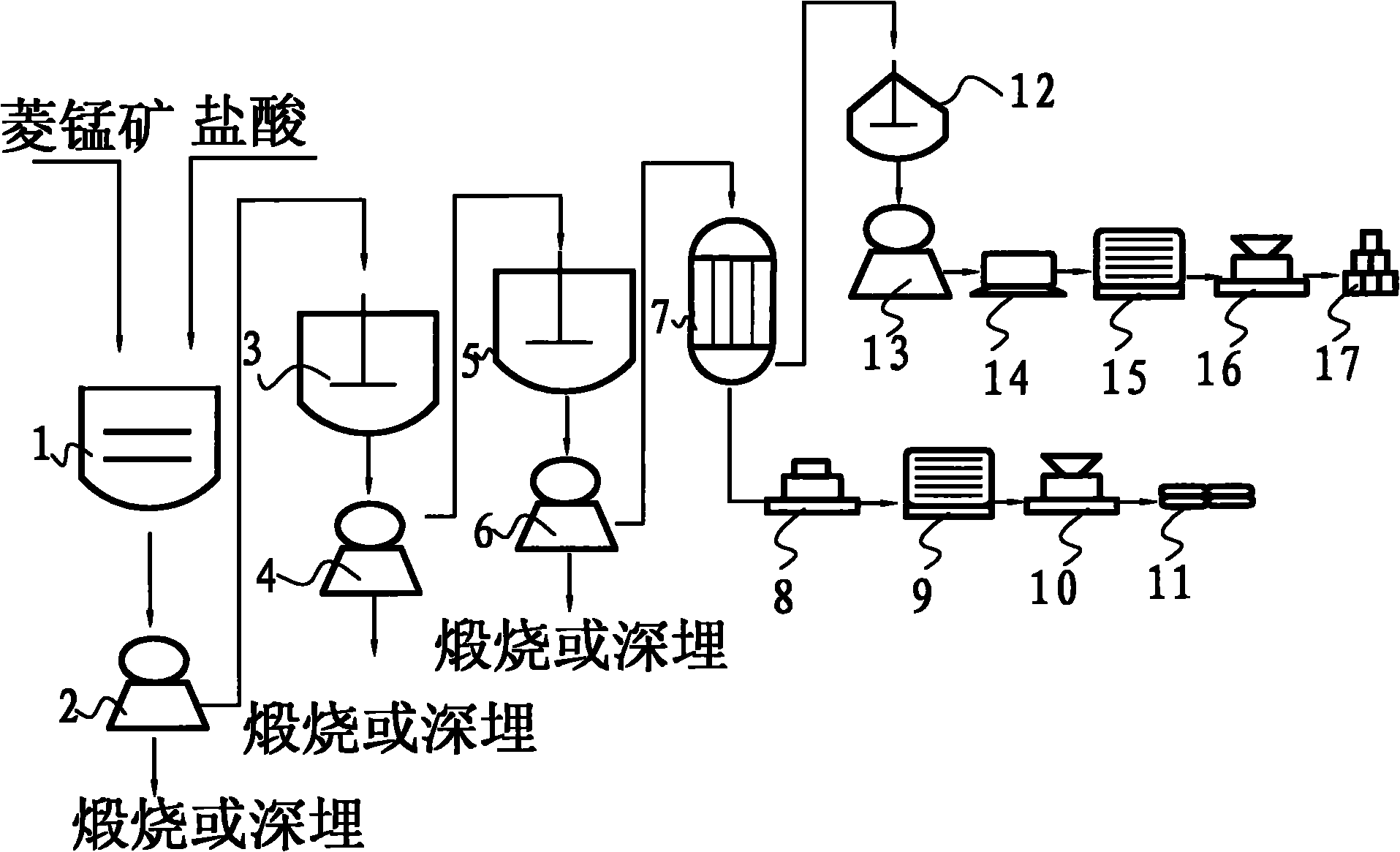
[0046] Using rhodochrosite to process waste sulfuric acid to prepare high-purity manganese nitrate and produce gypsum, the preparation steps are as follows:
[0047] Step A, 800kg rhodochrosite is pulverized into 100 mesh coarse powder;
[0048] Step B, putting the rhodochrosite coarse powder into the leaching tank 1 equipped with hydrochloric acid, and leaching for 8 hours with a pure mass ratio of 1:0.63; The concentration is 17%;
[0049] Step C, filtering the solid-liquid mixture leached in step B with the first filter 2, and calcining or deeply burying the filter cake impurities;
[0050]Step D, the filtrate obtained by filtering in step C and sulfuric acid are successively dropped into the first anticorrosion reactor 3 with a pure mass ratio of 1:0.78 to carry out stirring reaction, and the pure mass ratio of the filtrate to calcium hydroxide is according to the pure manganese chloride in the filtrate and sulfuric acid for conversion; before the filtrate is reacted wit...
Embodiment 3
[0058] Using rhodochrosite to process waste sulfuric acid to prepare high-purity manganese nitrate and produce gypsum, the preparation steps are as follows:
[0059] Step A, 800kg rhodochrosite is pulverized into 120 purpose coarse powder;
[0060] Step B, putting the rhodochrosite coarse powder into the leaching tank 1 equipped with hydrochloric acid, and leaching for 10 hours with a pure mass ratio of 1: 1.83; wherein, the rhodochrosite coarse powder (calculated as containing 13% manganese), the volume of The concentration is 20%;
[0061] Step C, filtering the solid-liquid mixture leached in step B with the first filter 2, and calcining or deeply burying the filter cake impurities;
[0062] Step D, the filtrate obtained by filtering in step C and sulfuric acid are successively dropped into the first anticorrosion reactor 3 with a pure mass ratio of 1:0.98 to carry out stirring reaction, and the pure mass ratio of the filtrate to calcium hydroxide is according to the pure m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com